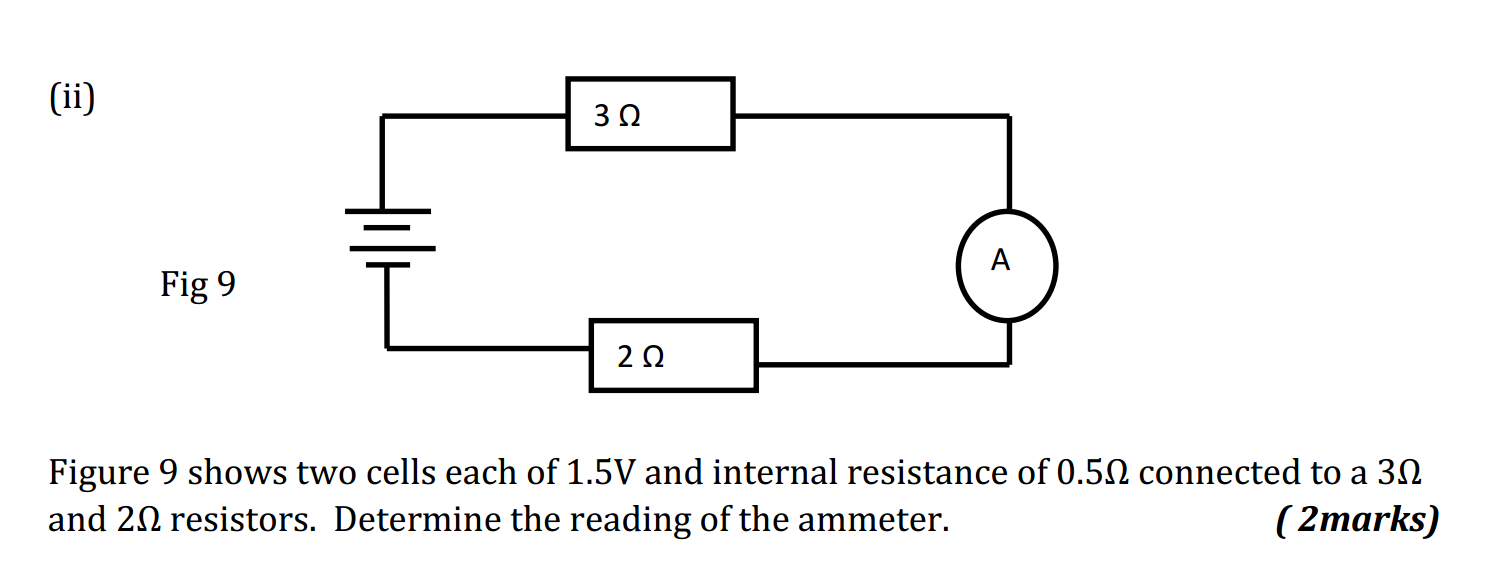
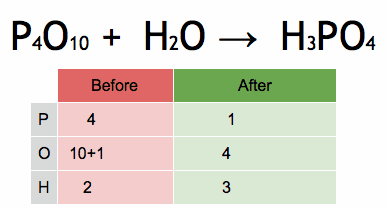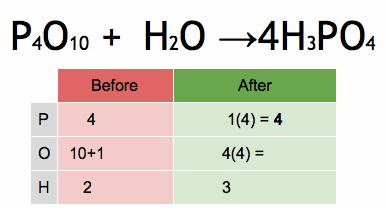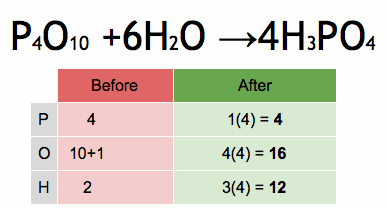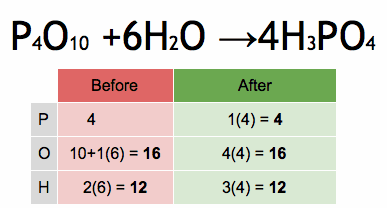
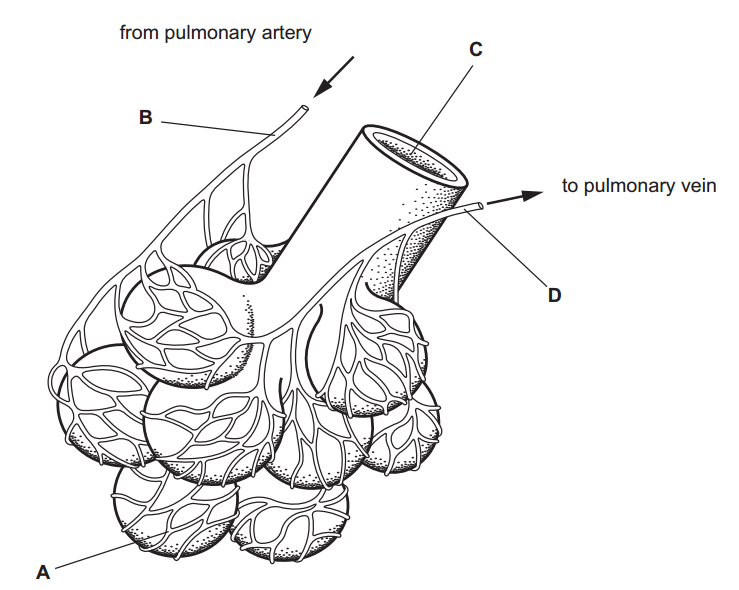
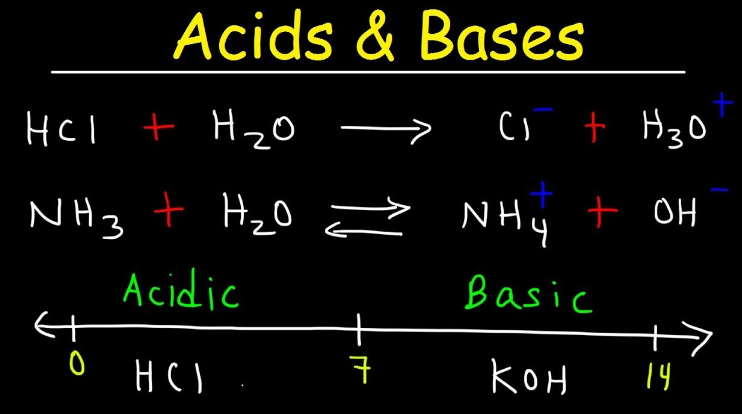
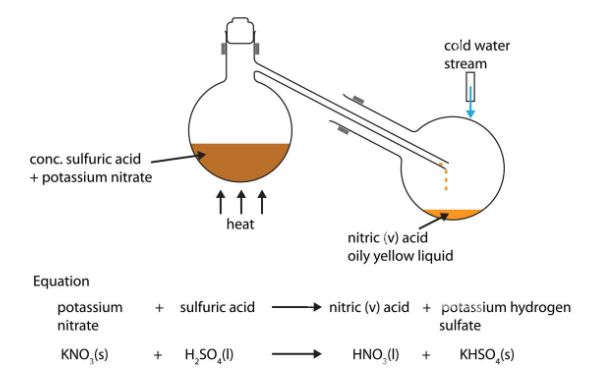
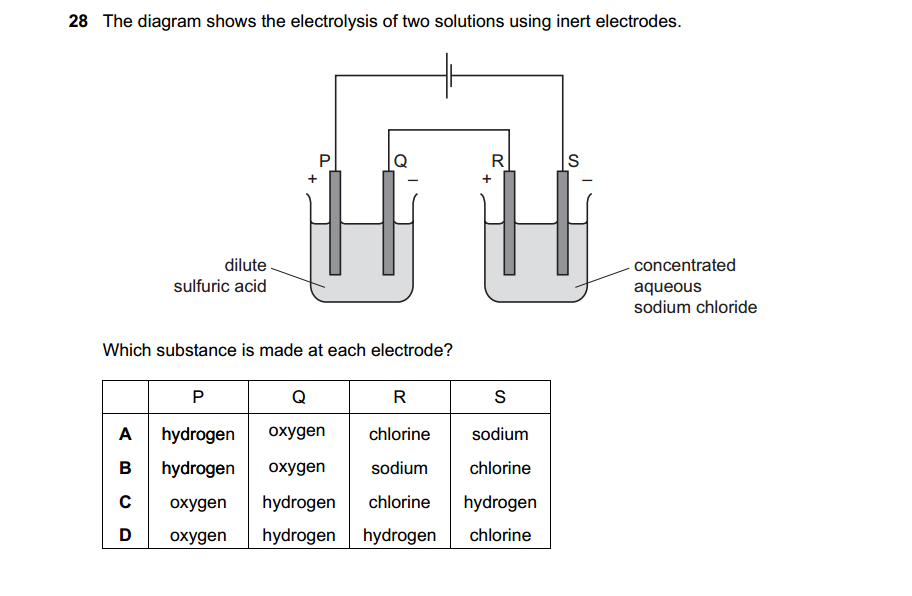
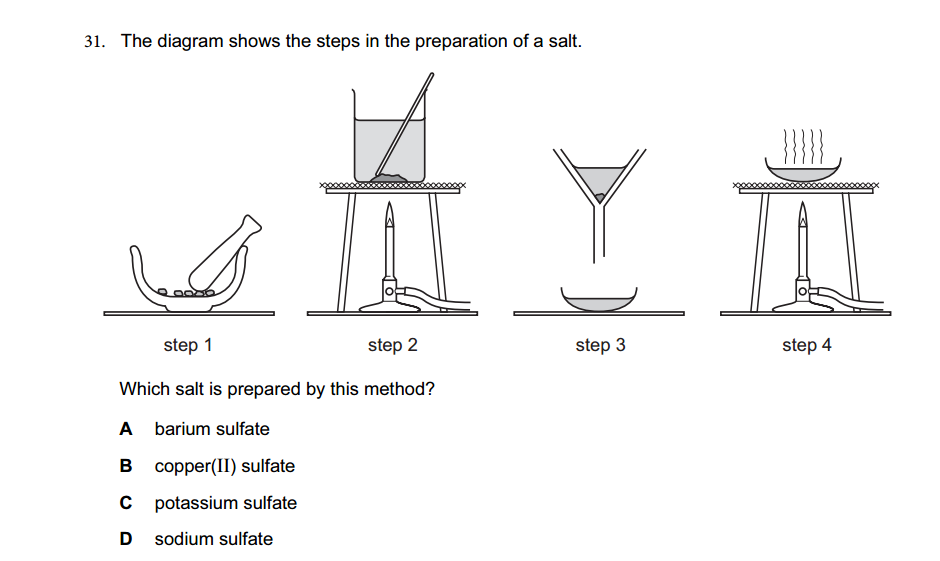
1.What is the definition of an acid according to the Arrhenius theory? a) A substance that donates protons (H⁺ ions) in aqueous solution b) A substance that accepts protons (H⁺ ions) in aqueous solution c) A substance that donates hydroxide ions (OH⁻) in aqueous solution d) A substance that accepts hydroxide ions (OH⁻) in aqueous solution 2.Which of the following is a characteristic property of acids? a) They turn litmus paper blue b) They taste bitter c) They react with metals to produce hydrogen gas d) They feel slippery to the touch 3.What is the pH value of a neutral solution at 25°C? a) 0 b) 7 c) 14 d) 10 4.Which of the following is a strong acid? a) Acetic acid (CH₃COOH) b) Hydrochloric acid (HCl) c) Carbonic acid (H₂CO₃) d) Citric acid (C₆H₈O₇) 5.Which of the following is a weak base? a) Sodium hydroxide (NaOH) b) Potassium hydroxide (KOH) c) Ammonia (NH₃) d) Calcium hydroxide (Ca(OH)₂) 6.What is the pH of a solution with a hydrogen ion concentration of 1 × 10⁻³ M? a) 1 b) 3 c) 7 d) 11 7.Which of the following is an example of a Lewis acid? a) HCl b) NaOH c) Fe³⁺ d) NH₃ 8.What is the product when an acid reacts with a base? a) Salt and water b) Salt and hydrogen gas c) Water and carbon dioxide d) Water and oxygen gas 9.What is the formula of the conjugate base of HCO₃⁻? a) HCO₃²⁻ b) CO₃²⁻ c) H₂CO₃ d) H₂O 10.Which of the following is a characteristic property of bases? a) They turn litmus paper red b) They taste sour c) They react with carbonates to produce carbon dioxide gas d) They conduct electricity in aqueous solution
ANSWERS
- a) A substance that donates protons (H⁺ ions) in aqueous solution
- c) They react with metals to produce hydrogen gas
- b) 7
- b) Hydrochloric acid (HCl)
- c) Ammonia (NH₃)
- b) 3
- c) Fe³⁺
- a) Salt and water
- b) CO₃²⁻
- c) They react with carbonates to produce carbon dioxide gas