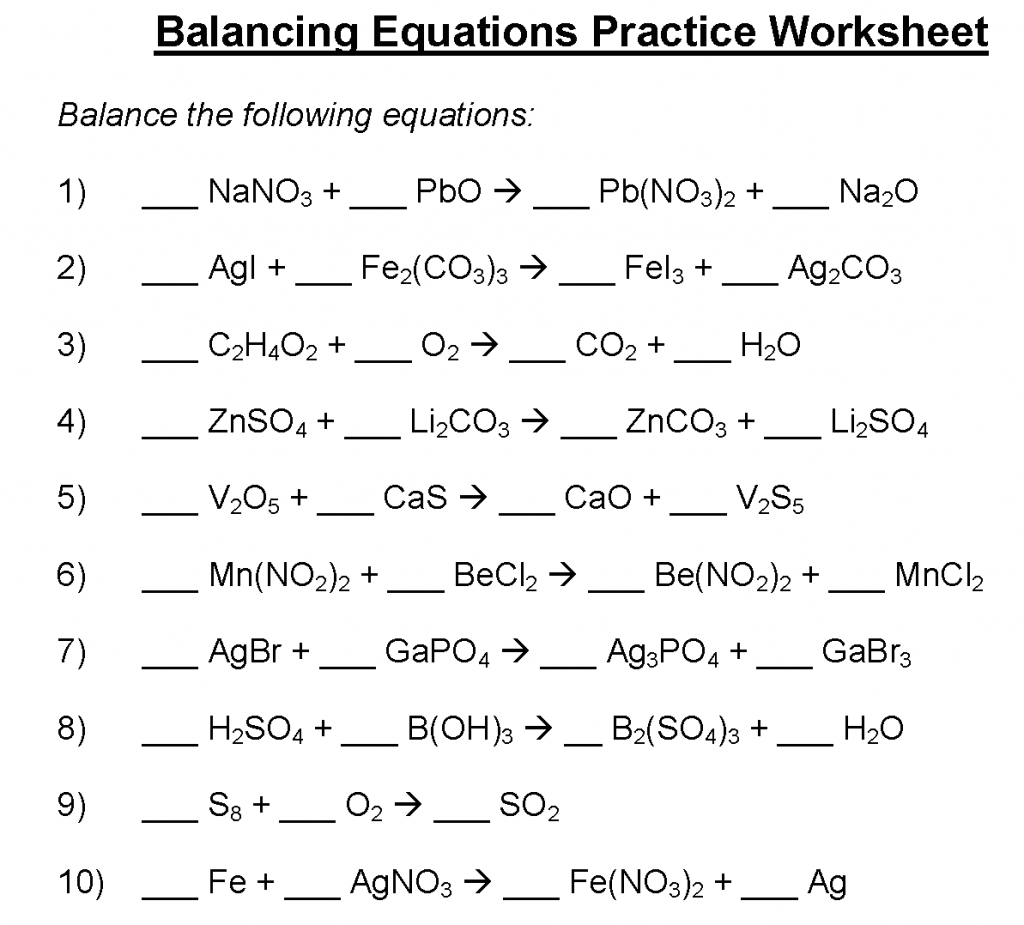
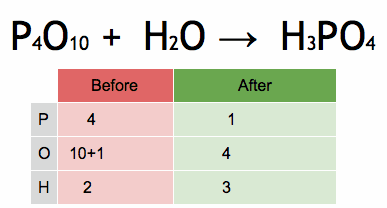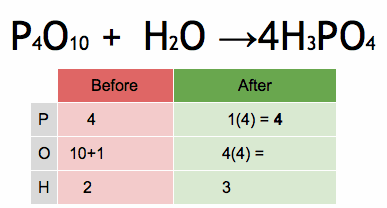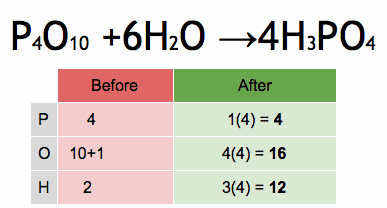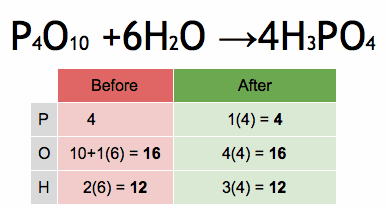
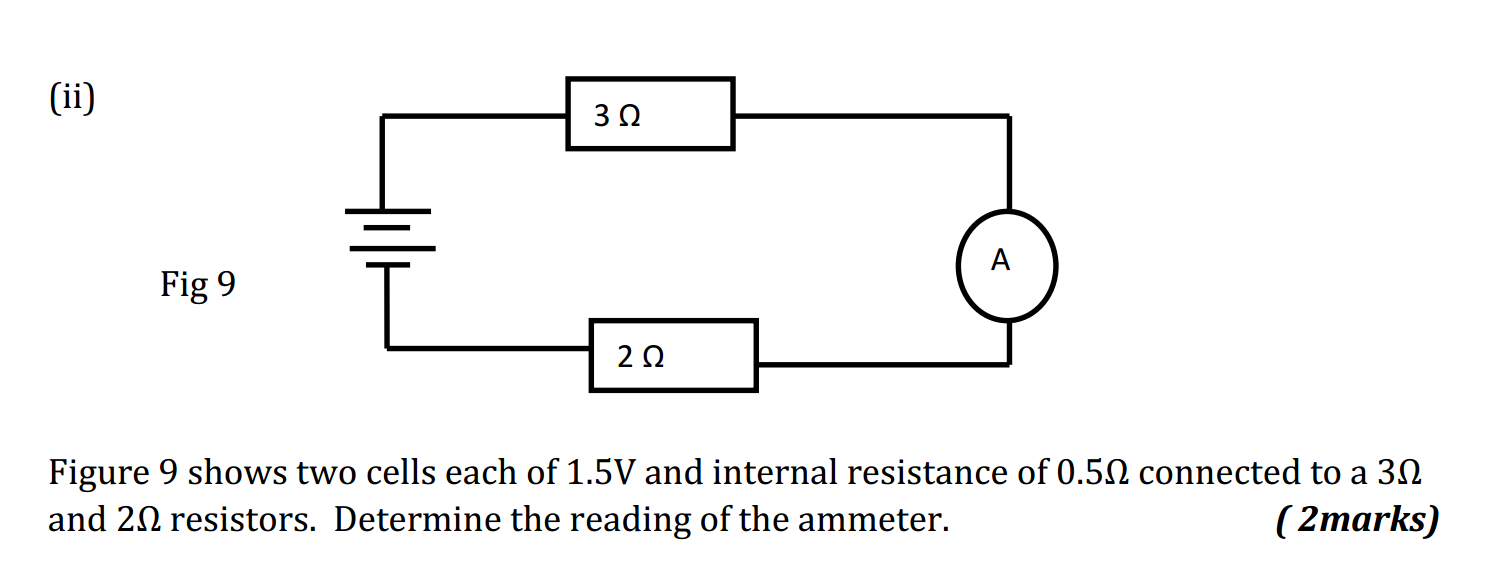
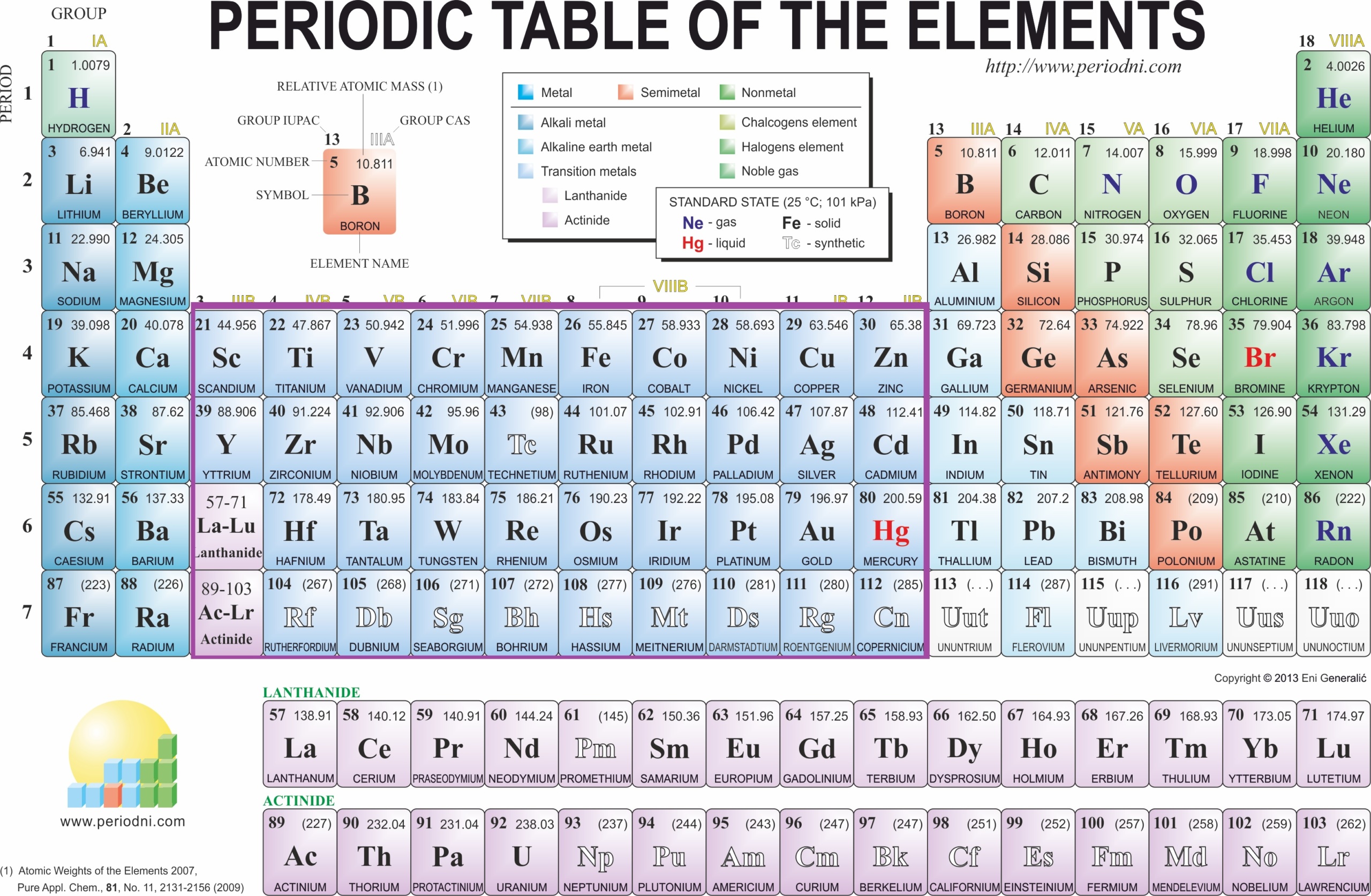
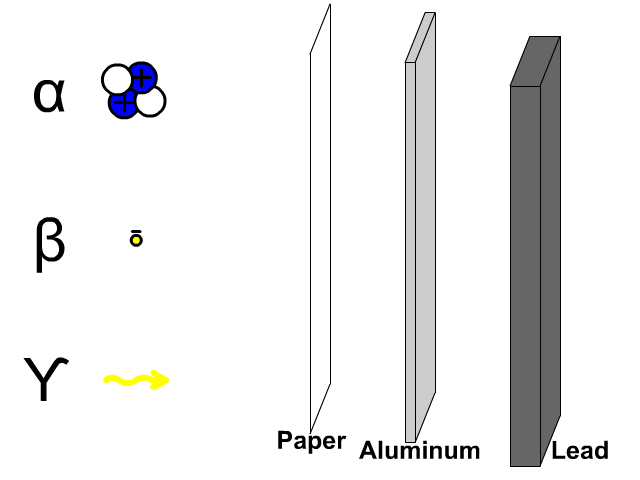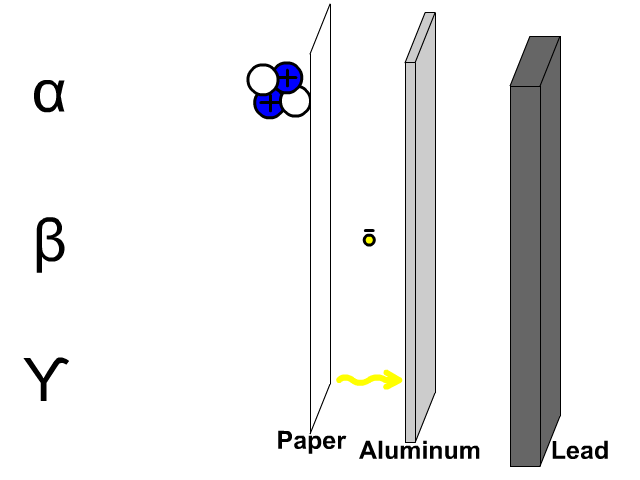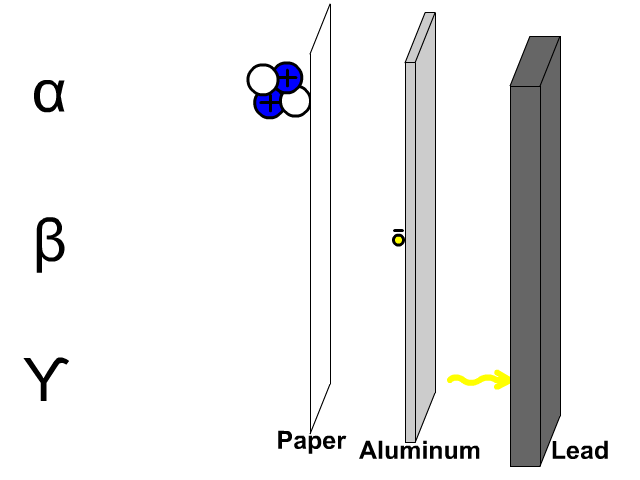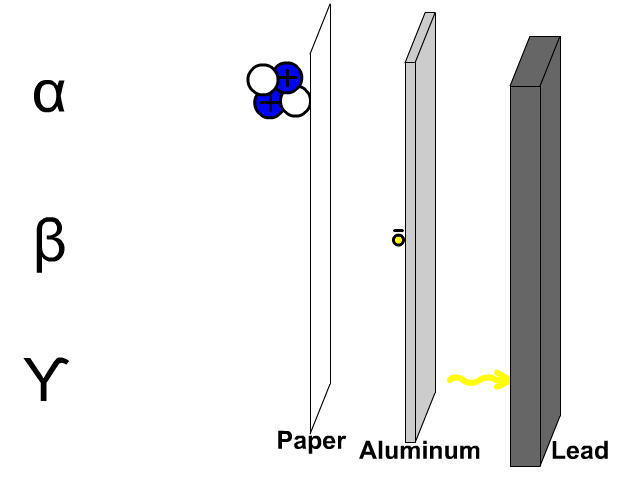
- What is the chemical symbol for oxygen?
- Define an element in chemistry.
- How many valence electrons does carbon have?
- Name two allotropes of carbon.
- What is the formula of sulfuric acid?
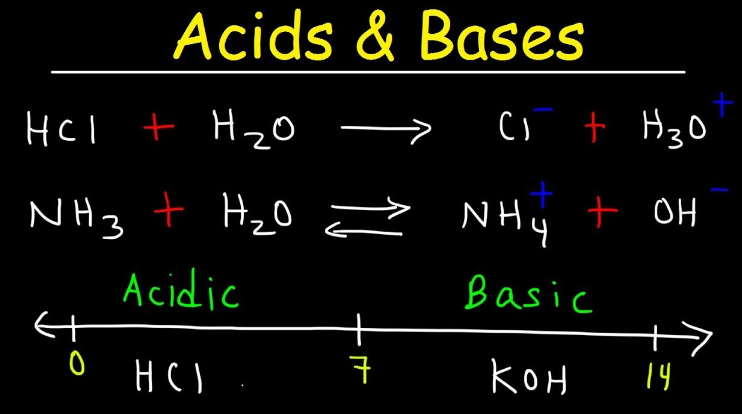
- Define an acid in chemistry.
- What is the pH range of acidic solutions?
- What are the products of the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH)?
- What is the name of the process by which solid changes directly to a gas without becoming a liquid first?
- What are the products of the complete combustion of a hydrocarbon in excess oxygen?
- Define the term “exothermic reaction.”
- What type of bond forms between two nonmetals?
- What is the balanced chemical equation for the reaction between magnesium (Mg) and oxygen (O2)?
- Name the gas produced when acids react with metals.
- Define a precipitate in a chemical reaction.
- What is the formula of ammonium sulfate?
- How many electrons can the first energy level (n=1) hold?
- What is the formula of potassium nitrate?
- Define a catalyst in a chemical reaction.
- What is the molecular formula of ethanol?
- Name two gases that cause acid rain.
- Define an exothermic reaction.
- What is the formula of calcium carbonate?
- How many atoms are present in one molecule of water (H2O)?
- Define the term “endothermic reaction.”
- Name the process by which a solid changes directly to a gas without becoming a liquid first.
- What is the molecular formula of glucose?
- Define the term “reduction” in chemistry.
- What is the chemical symbol for sodium?
- What is the formula of nitric acid?
- Define a base in chemistry.
- Name the gas produced when acids react with carbonates.
- What is the balanced chemical equation for the reaction between hydrochloric acid (HCl) and calcium carbonate (CaCO3)?
- Define the term “activation energy.”
- What is the molecular formula of methane?
- What is the formula of sodium chloride?
- Define an endothermic reaction.
- Name the process by which a gas changes directly to a solid without becoming a liquid first.
- What is the formula of sulfur dioxide?
- Define the term “oxidation” in chemistry.
- What is the chemical symbol for iron?
- Name the type of bond that forms between a metal and a nonmetal.
- What is the balanced chemical equation for the reaction between sulfuric acid (H2SO4) and potassium hydroxide (KOH)?
- Define the term “valency” in chemistry.
- What is the molecular formula of ethane?
- What is the formula of hydrochloric acid?
- Define the term “neutralization reaction.”
- Name the gas produced when acids react with metal carbonates.
- What is the chemical symbol for copper?
- What is the formula of sodium hydroxide?
- Define the term “molecular formula” in chemistry.
- What is the molecular formula of propane?
- What is the formula of nitrous acid?
- Define the term “homologous series” in organic chemistry.
- What is the chemical symbol for silver?
- Name the gas produced when acids react with metal sulfites.
- What is the formula of hydrofluoric acid?
- Define the term “electrolysis” in chemistry.
- What is the molecular formula of butane?
- What is the formula of sulfur hexafluoride?
- Define the term “atomic number” in chemistry.
- What is the formula of acetic acid?
- Name the gas produced when acids react with metal sulfides.
- What is the chemical symbol for gold?
- What is the molecular formula of pentane?
- Define the term “isotope” in chemistry.
- What is the formula of hydrobromic acid?
- Name the gas produced when acids react with metal sulfates.
- What is the chemical symbol for zinc?
- What is the molecular formula of hexane?
- Define the term “mole” in chemistry.
- What is the formula of hydroiodic acid?
- Name the gas produced when acids react with metal oxides.
- What is the chemical symbol for chlorine?
- What is the molecular formula of heptane?
- Define the term “stoichiometry” in chemistry.
- What is the formula of hydrosulfuric acid?
- Name the gas produced when acids react with metal hydroxides.
- What is the chemical symbol for nitrogen?
- What is the molecular formula of octane?
- Define the term “molar mass” in chemistry.
- What is the formula of phosphoric acid?
- Name the gas produced when acids react with metal hydrides.
- What is the chemical symbol for carbon?
- What is the molecular formula of ethene?
- Define the term “stoichiometric ratio” in chemistry.
- What is the formula of carbonic acid?
- Name the gas produced when acids react with metal bicarbonates.
- What is the chemical symbol for oxygen?
- What is the molecular formula of propene?
- Define the term “Avogadro’s number” in chemistry.
- What is the formula of perchloric acid?
- Name the gas produced when acids react with metal bicarbonates.
- What is the chemical symbol for hydrogen?
- What is the molecular formula of butene?
- Define the term “empirical formula” in chemistry.
- What is the formula of nitrous acid?
- Name the gas produced when acids react with metal bicarbonates.
- What is the chemical symbol for helium?
- What is the molecular formula of pentene?
Please note that these questions cover a wide range of topics in O level Chemistry and can be used for practice and review.