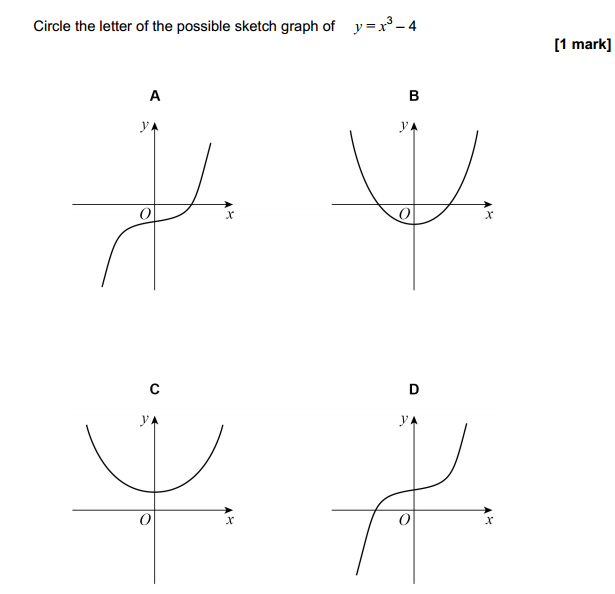
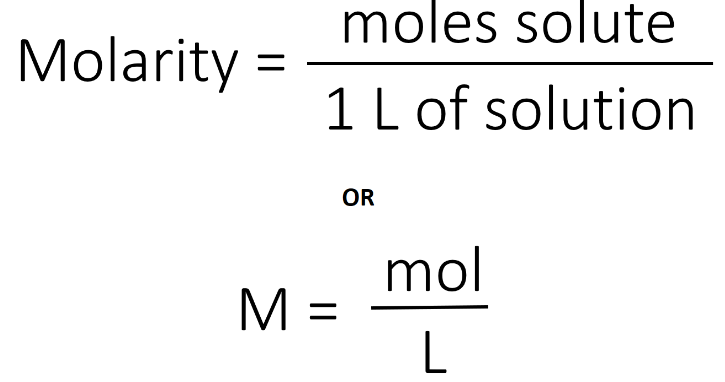1 (a) Complete the following equations.

(b) An element Y has three naturally occurring isotopes with isotopic masses and relative
abundances as shown below:

Calculate the average atomic mass of Y.
2. Complete the following equations and outline the mechanism in each case.


(c) Using a suitable reagent state how the following can be differentiated. Include the
observation and a suitable equation for the reaction.
C3H6 and C2H4.
3 (a) Define the following;
(i) Enthalpy of combustion.
(ii) Enthalpy of reaction.
(b) Given the following thermo chemical data.

Determine the standard enthalpy of formation of ethyne.
4 a) Write the electronic configuration of manganese. (Atomic number = 25)
b) Explain why manganese
(i) is a transition metal.
(ii) acts as a catalyst.
(iii) forms complex ions.
c) Describe the reaction of manganese with
(i) water (02marks)
(ii) sulphuric acid (04marks)
d) Describe the reaction of manganese
(II) chloride with
(i) sodium hydroxide solution (05marks)
(ii) a mixture of nitric acid and sodium bismuthate (03marks)
5. Write equations to show how the following compounds can be synthesized. Indicate the reagents and
conditions


6.Explain the following observations (write equations where necessary)
a) when acidified potassium manganate (VII) solution is added to aqueous potassium iodide
solution, the solution turns brown.
b) When iron (III) chloride solution is added to an aqueous solution of sodium hydrogen
carbonate a colourless gas and a brown solid is formed.
c) Phenolphthalein indicator pH range 8 – 10, is a suitable indicator in the titration of ethanoic acid with sodium hydroxide solution but not suitable for ammonia solution with hydrochloric
acid.
d) Lithium carbonate decomposes when heated strongly whereas sodium carbonate does not.
d) Lithium carbonate decomposes when heated strongly whereas sodium carbonate does not.
7.a) When hydrogen iodide is heated it decomposes according to the equation

(i) Describe an experiment to determine the equilibrium constant of the reaction. (07marks)
(ii) 3 moles of hydrogen and one mole of iodine were heated together at 500°C until equilibrium
was attained. Calculate the number of moles of hydrogen iodide present in the equilibrium mixture at 500°C. (The equilibrium constant, Kc for the reaction between hydrogen and iodine is 50) (05marks)
b) Explain how the position of the equilibrium, value of the equilibrium constant and the rate of
attainment of equilibrium would be affected if;
(i) the temperature of the reaction was increased. (03marks)
(ii) the pressure of the reaction was decreased. (03marks)
8. a) Explain the trend in the boiling points of group (VII) elements. (03marks)
b) Explain why hydrogen fluoride
(i) is a weaker acid in dilute aqueous solution than in concentrated solution. (03marks)
(ii) has a higher boiling point than hydrogen iodide. (03marks)
c) Write equation for the reaction between hydrogen fluoride and silicon (IV) oxide. (01mark)
d) Describe the reactions of group (VII) elements with sodium hydroxide. (07marks)
e) Write the equation for the reaction between sulphuric acid and
(i) sodium chloride
(ii) potassium bromide
(iii) sodium iodide (03marks)
9. Complete the following equations and in each case outline a mechanism for the reaction.







10.(a) Write the outermost electronic configuration of group(II)
elements.
(b) Describe the reactions of group(II) elements with:
(i) Water
(ii) sulphuric acid
(iii) Sodium hydroxide (15 marks)
(c) Potassium chromate solution was added to barium chloride solution
followed by dilute nitric acid drop-wise until in excess.
(i) State what was observed.
(ii) Write equation(s) for the reaction(s) that took place.
(04marks)
11.An organic compound , Q contains carbon , hydrogen and oxygen only. On
complete combustion in excess oxygen , 1 mole of Q produced 2 moles of
carbon dioxide and 3 moles of water. When vaporized at 101kPa and 373K ,
0.1g of X occupied a volume of 66.7cm3.
(a) (i) Determine the molecular formula of Q. (04marks)
(ii) Write structural formulae and names of the possible isomers of Q.
(03marks).
(b) When Q was treated with iodine solution in the presence of sodium
hydroxide solution , a yellow precipitate was formed on warming. Identify
Q and the yellow precipitate. (01mark)
(c) Write an equation to how the yellow precipitate was formed.
(01mark)
(d) Discuss the reactions of Q with phosphoric acid. In each case outline
mechanism for the reaction. (11marks)
12.Explain each of the following observations.
(a) Both aluminium oxide and silicon(IV) oxide dissolve in concentrated
sodium hydroxide solution. (04marks)
(b) Ethylamine is a stronger base than phenyl amine although both are
primary amines. (04marks)
(c) An aqueous solution of ethanol boils at 78.2oC where as an aqueous
solution of sodium chloride boils at 106oC at 760mmHg. (04marks)
(d) When sodium hydroxide solution was added to cobalt(II) sulphate
solution drop-wise until in excess blue precipitate was formed which was
insoluble in excess and the precipitate turned brown on standing .
(04marks)
(e) When excess concentrated hydrochloric acid was added to lead(IV) oxide
, dark brown solid dissolved to form a bright yellow solution which
formed yellow precipitate on addition of a saturated solution of
ammonium chloride.
13.(a) (i) What is meant by the term ore? (01mark)
(ii) Write the formula and name of one ore of iron apart from spathic
iron ore from which iron can be extracted. (01mark)
(b) Describe how cast iron is extracted from spathic iron ore.
(Diagram not required.) (7½marks)
(c) Describe the reaction of iron with
(i) water (3marks)
(ii) sulphuric acid (05marks)
(iii) bromine (2½marks)
14.a) (i) Define the term standard electrode potential. (02 marks)
(ii) Explain why it is not possible to measure standard electrode potential
absolutely. (02 marks)
(iii) State and explain the factors that affect the value of standard electrode
potential. (06 marks)
(b) Describe;
(i) a standard hydrogen half cell. (02 marks)
(ii) how you would measure standard electrode potential of a metal in a
solution of its ions. (03 marks)
(c) Some half cells and their emfs are given below:

calculate the emf of the cell. (03 marks)
(d) Explain whether the cell is feasible or not.
15. (a) Using equations, describe the reactions of zinc with
(i) air which is moist,
(ii) water,
(iii) sodium hydroxide. (10 marks)
(b) (i) Explain why zinc is not a typical transition metal. (02 marks)
(ii) Give four ways in which zinc reacts the same way as magnesium.
(04 marks)
(c) State what is observed and write equation(s) for the reaction when dilute
ammonia solution is added drop wise until in excess to a solution containing
zinc ions. (04 marks)
16.Propanone and propanal can both be prepared from propene.
(a) Give the reaction schemes indicating the reagents and conditions leading to the
preparation of the two carbonyl compounds starting with propene. (07 marks)
(b) Name one reagent that can be used to distinguish between the two carbonyl
compounds in (a). In each case, state what is observed when the reagent named
is used. (03 marks)
(c) Give a reaction scheme for the conversion of propanal to propanone.
(04 marks)
(d) Propanone was condensed with 2, 4 – dinitrophenyl hydrazine.
(i) State the observation made. (01 mark)
(ii) Name the product formed. (01 mark)
(iii) Suggest a mechanism for the reaction. (04 marks)