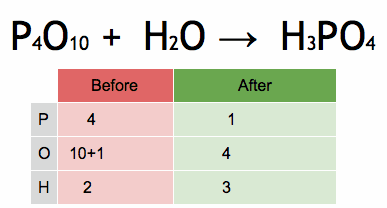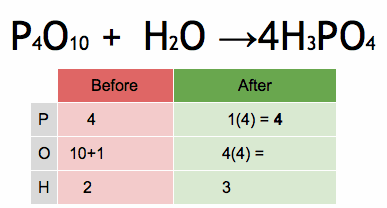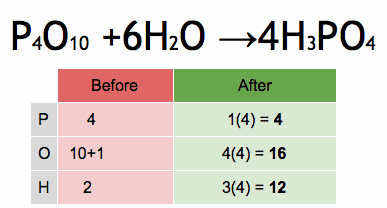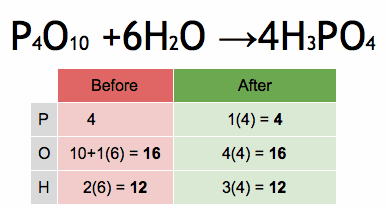
The enthalpy of a chemical reaction, often denoted as ΔH (delta H), is a measure of the heat energy absorbed or released during a chemical reaction at constant pressure. Enthalpy is a state function, which means it depends only on the initial and final states of the system, not on the path taken between those states.
There are two main components of enthalpy:
- Internal Energy (ΔU): This is the change in the internal energy of the system, which includes the kinetic and potential energy of the particles (atoms and molecules) that make up the system.
- Pressure-Volume Work (PΔV): This represents the work done by or on the system as a result of changes in volume at constant pressure. When a system expands or contracts, it can do work on its surroundings or have work done on it.
The enthalpy change for a chemical reaction can be calculated using the following equation:
ΔH = ΔU + PΔV
In practice, it’s often challenging to measure the internal energy change directly. Instead, chemists rely on experimental calorimetry to measure the heat exchange (q) during a reaction at constant pressure, and this heat exchange is equal to the change in enthalpy (ΔH) for the reaction.
If ΔH is positive, it indicates that the reaction is endothermic, meaning it absorbs heat from the surroundings. If ΔH is negative, it signifies that the reaction is exothermic, releasing heat into the surroundings.
Enthalpy is a fundamental concept in thermodynamics and is widely used in chemistry to understand and predict the heat changes associated with chemical reactions, making it essential for the study of chemical processes and their practical applications.
20 Discussion Questions on this topic.
- What is enthalpy in the context of a chemical reaction?
- How is enthalpy different from internal energy?
- Define the standard enthalpy of formation.
- How is enthalpy change (ΔH) related to heat exchange in a reaction?
- Explain the concept of endothermic and exothermic reactions with respect to enthalpy.
- How can you experimentally determine the enthalpy change for a reaction?
- What is the enthalpy of combustion, and how is it measured?
- How does the enthalpy of a reaction relate to the energy level diagram of the reaction?
- Define Hess’s Law and its significance in enthalpy calculations.
- What is the enthalpy of reaction for a chemical equation, and how is it calculated?
- How does the enthalpy of a reaction relate to bond energies?
- Describe the relationship between the enthalpy change and the direction of a chemical reaction.
- Can enthalpy change be negative, positive, or zero? What does each case represent?
- How does pressure affect the enthalpy change of a reaction?
- How is enthalpy used in the context of calorimetry?
- What is the role of enthalpy in the Gibbs free energy equation and thermodynamic spontaneity?
- Explain the enthalpy change for phase transitions, such as melting and boiling.
- How is the enthalpy change for a reaction affected by temperature changes?
- How can enthalpy be used to calculate the heat of solution for a solute in a solvent?
- What are some real-world applications of understanding and manipulating enthalpy changes in chemical reactions?