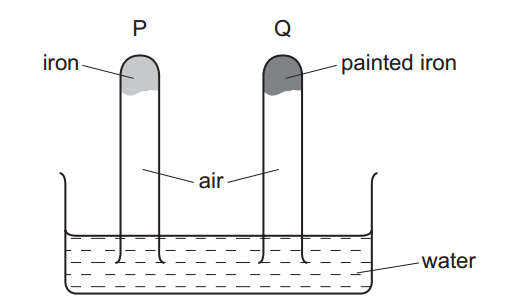
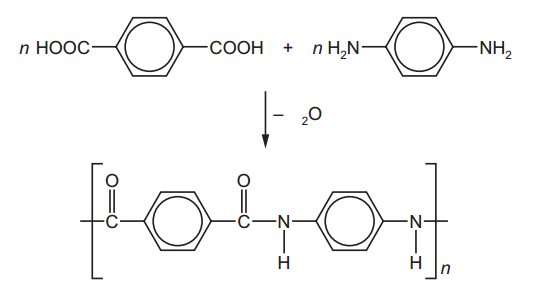
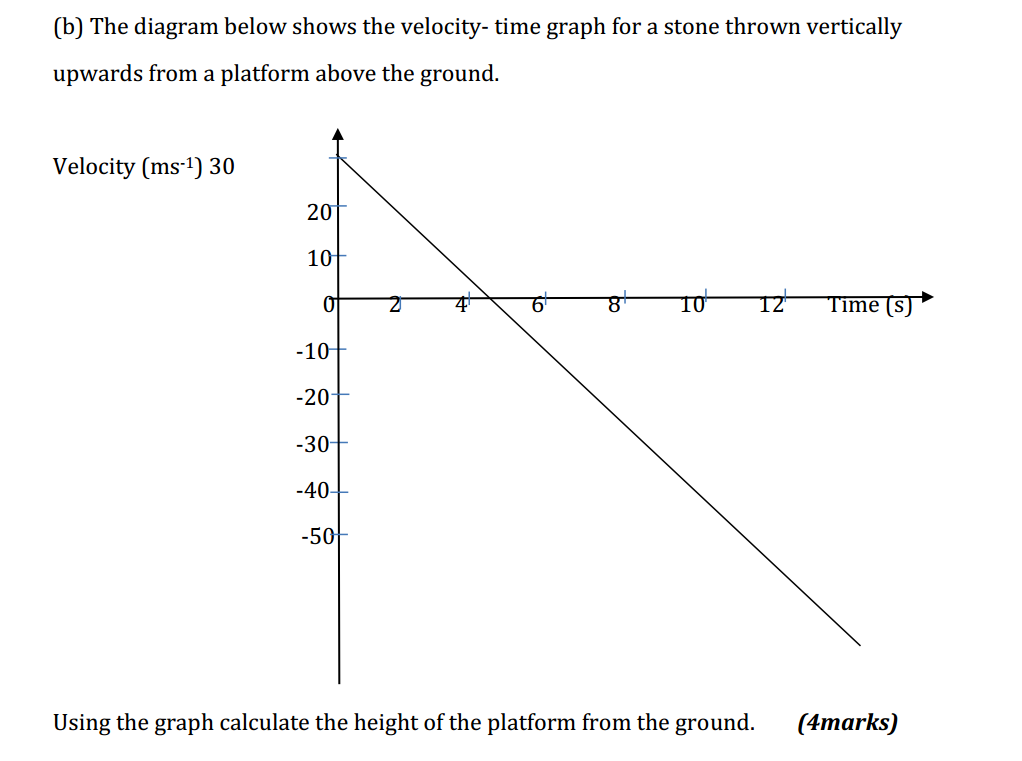
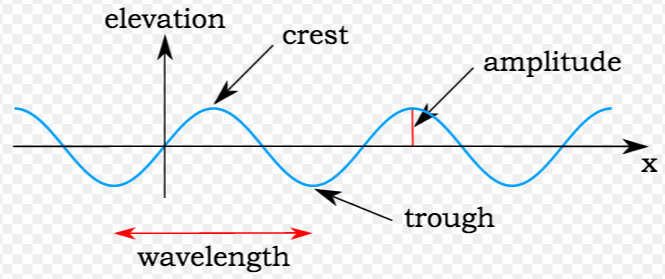
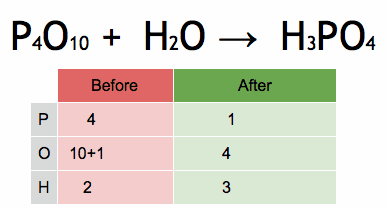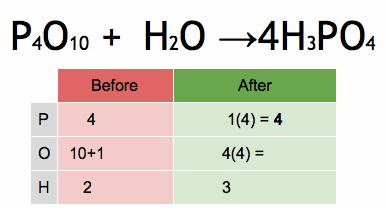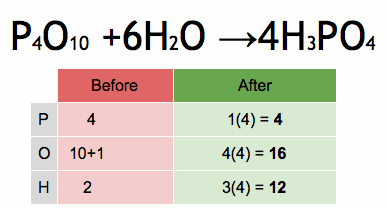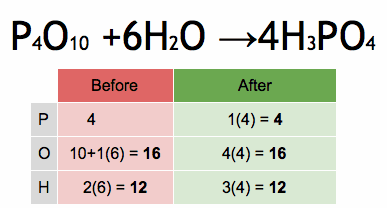
The reactivity series, also known as the activity series, is a list of metals arranged in order of their reactivity. In this series, metals are ranked from the most reactive to the least reactive. The reactivity of a metal is determined by its tendency to lose electrons and form positive ions when it reacts with other substances, typically with acids or oxygen.
The reactivity series is a useful tool in chemistry and is often used to predict how different metals will behave in various chemical reactions. It helps in understanding which metals will readily react with acids to produce hydrogen gas, for example, or which metals will corrode when exposed to air and moisture.
Here is a simplified reactivity series with some common metals, listed from the most reactive to the least reactive:
- Potassium (K)
- Sodium (Na)
- Calcium (Ca)
- Magnesium (Mg)
- Aluminum (Al)
- Zinc (Zn)
- Iron (Fe)
- Lead (Pb)
- Hydrogen (H)
- Copper (Cu)
- Silver (Ag)
- Gold (Au)
Metals at the top of the reactivity series (e.g., potassium and sodium) are highly reactive and readily react with water and acids. They can displace hydrogen from acids and are referred to as “active” metals. Metals at the bottom of the series (e.g., copper, silver, and gold) are less reactive and do not readily react with acids or water. They are often referred to as “noble” metals.
The reactivity series is a valuable tool in understanding the behavior of metals in various chemical reactions and is frequently used in teaching and laboratory experiments. However, it’s important to note that there are more comprehensive and detailed versions of the reactivity series that include a wider range of metals and account for specific reactions and conditions.