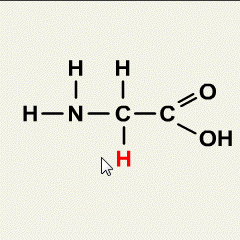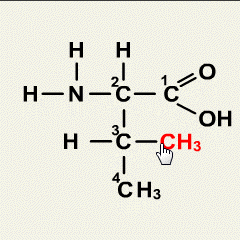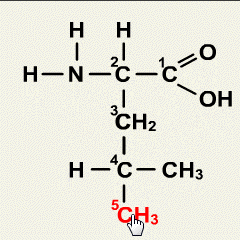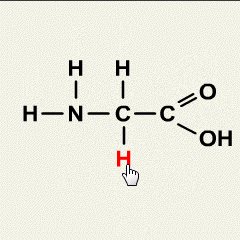
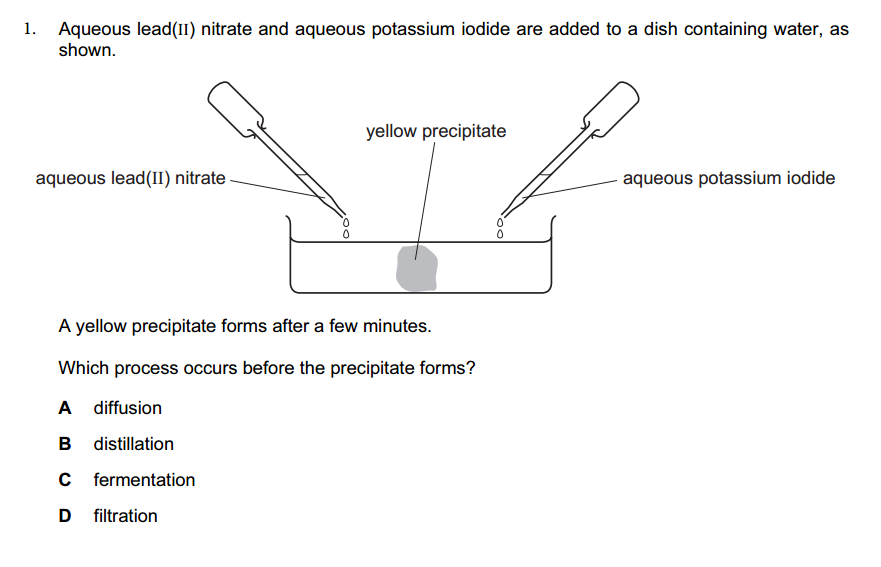
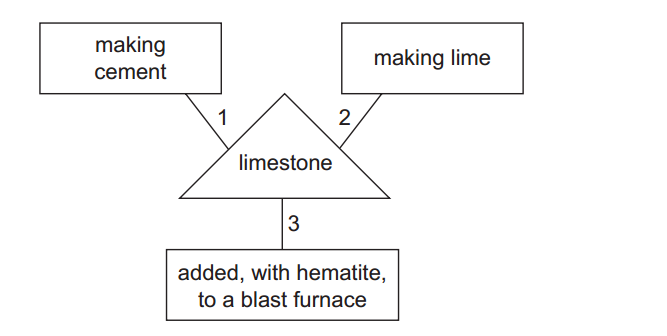
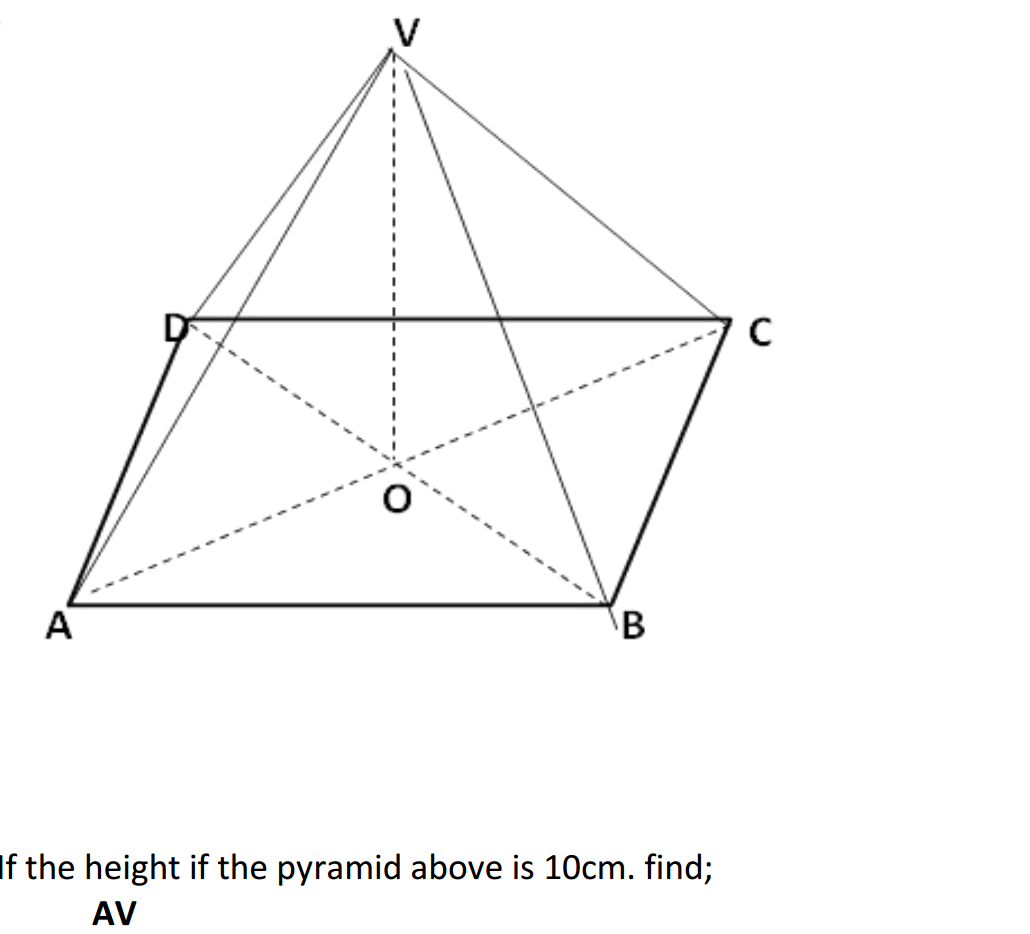
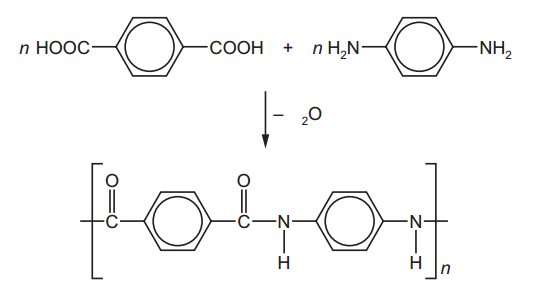
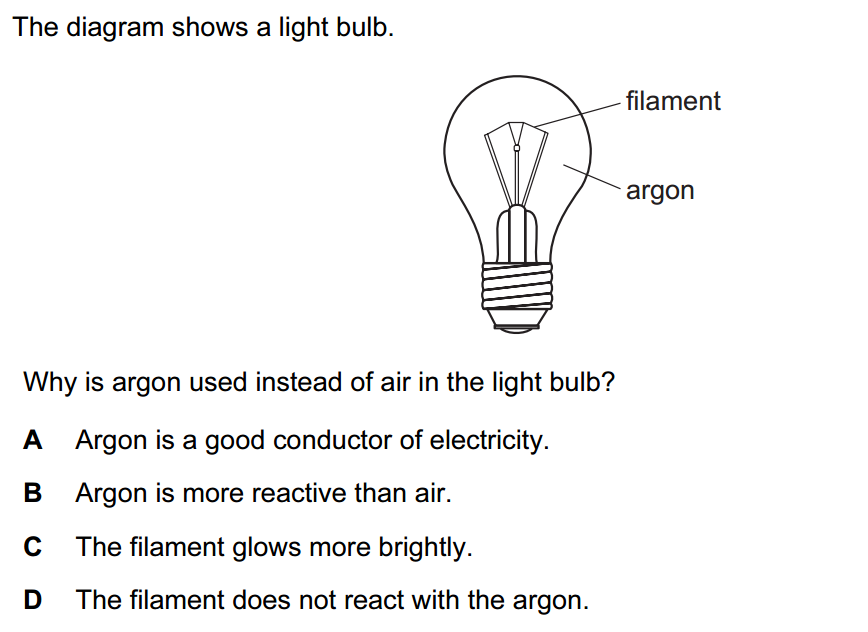
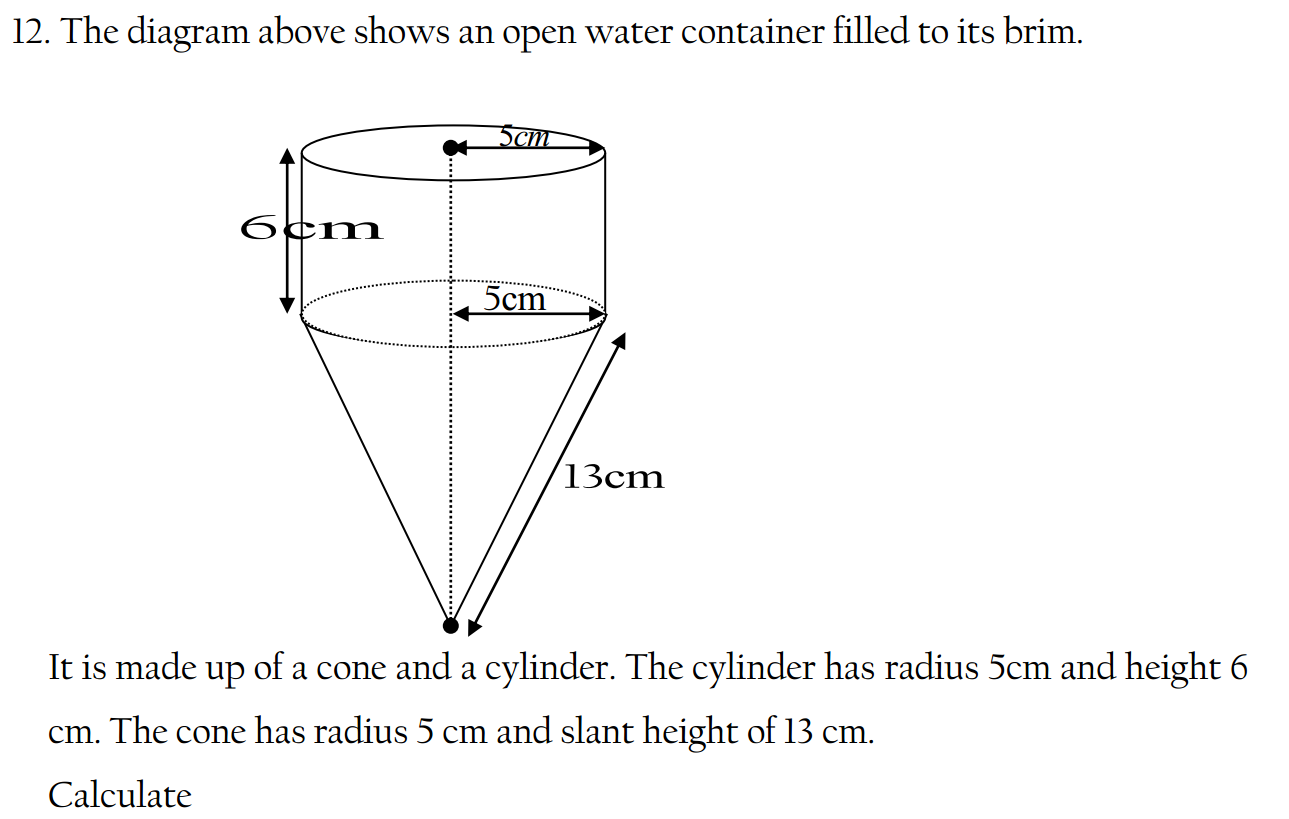
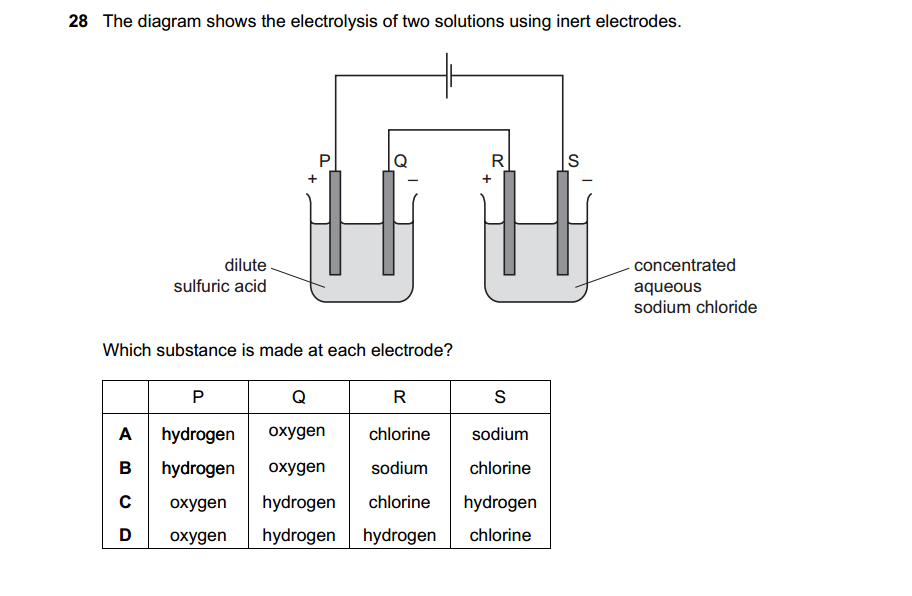
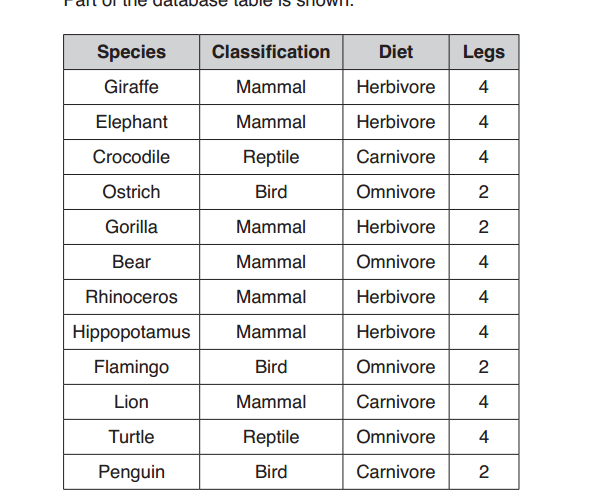
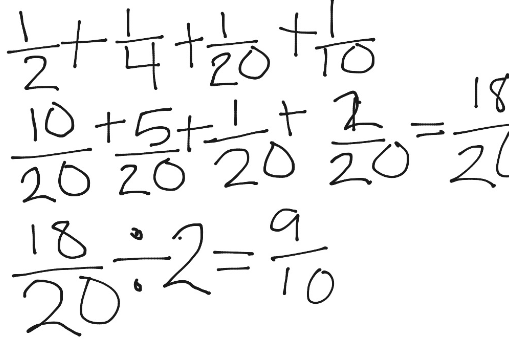- Rock salt is a mixture of sand and sodium chloride.
Sodium chloride is soluble in water but not in hexane.
Sand is insoluble in both water and hexane.
What is required to separate the sand from the sodium chloride?
1 filter paper
2 fractionating column
3 hexane
4 water
A. 1 and 3 B. 1 and 4 C. 2 and 3 D. 2 and 4. - 2. Calcium reacts with chlorine to produce calcium chloride.
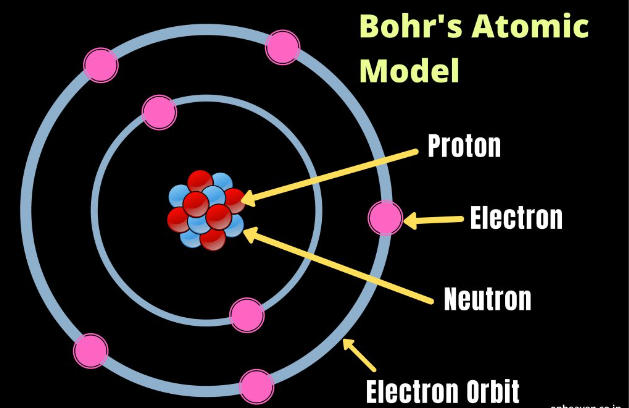
- What happens when a calcium ion forms during this reaction?
- A The calcium atom gains one electron.
- B The calcium atom gains two electrons.
- C The calcium atom loses one electron.
- D The calcium atom loses two electrons.
2. Calcium reacts with chlorine to produce calcium chloride.
What happens when a calcium ion forms during this reaction?
A The calcium atom gains one electron.
B The calcium atom gains two electrons.
C The calcium atom loses one electron.
D The calcium atom loses two electrons.
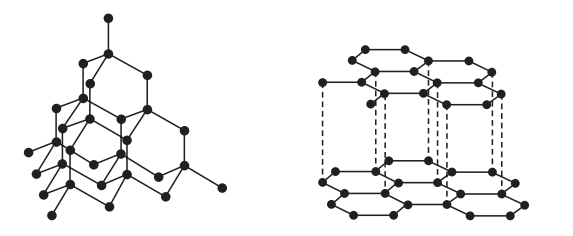
3. Diamond and graphite have giant covalent structures of carbon atoms.
Which statement describes graphite?
A It has a strong, rigid three-dimensional structure.
B It has four strong covalent bonds between each carbon atom.
C It has layers, which can slide over each other.
D It has no free electrons, so does not conduct electricity.
4. Which process is a physical change?
A burning wood
B cooking an egg
C melting an ice cube
D rusting iron
5. When blue-green crystals of nickel(II) sulfate are heated, water is produced and a yellow solid
remains. When water is added to the yellow solid, the blue-green colour returns.
Which process describes these changes?
A combustion
B corrosion
C neutralisation
D reversible reaction
6. How could crystals of a pure salt be prepared from dilute sulfuric acid?
A add an excess of aqueous sodium hydroxide, filter, evaporate the filtrate to crystallisation
point
B add an excess of copper(II) carbonate, filter, evaporate the filtrate to dryness
C add an excess of copper metal, filter, evaporate the filtrate to crystallisation point
D add an excess of zinc oxide, filter, evaporate the filtrate to crystallisation point
7. Why is ethanol a member of the homologous series of alcohols but propane is not?
A Ethanol has two carbon atoms per molecule but propane has three.
B Ethanol can be made from ethene but propane is obtained from petroleum.
C Ethanol is a liquid but propane is a gas.
D Ethanol contains the same functional group as other alcohols but propane does not.
The meeting of two personalities is like the contact of two chemical substances: if there is any reaction, both are transformed.
Carl Gustav Jung