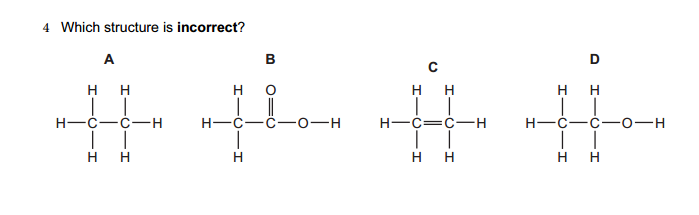
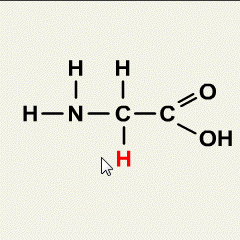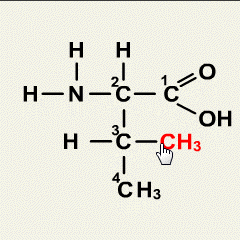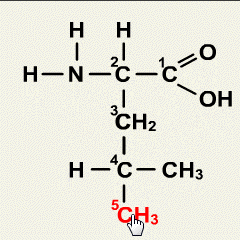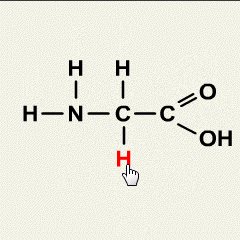
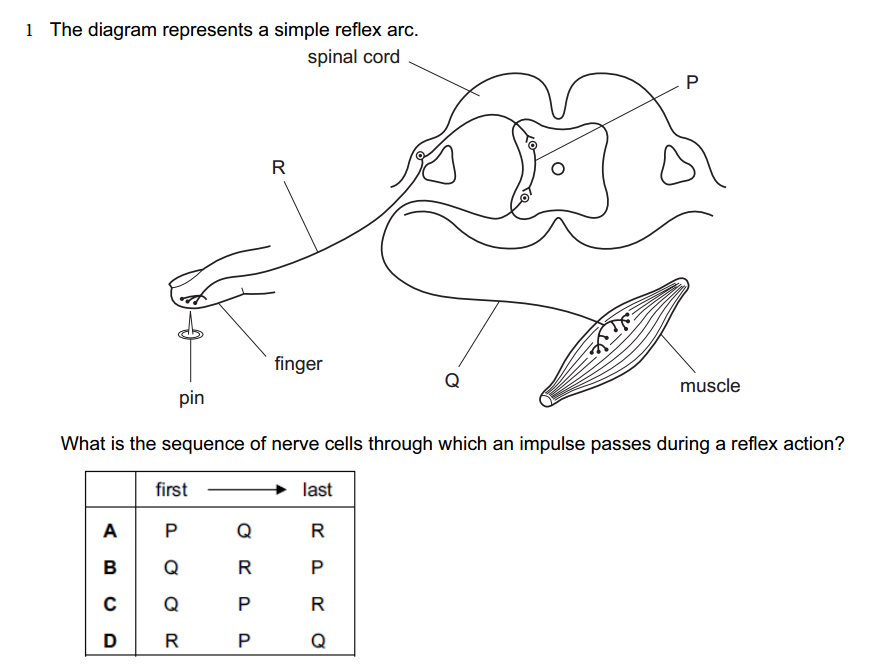
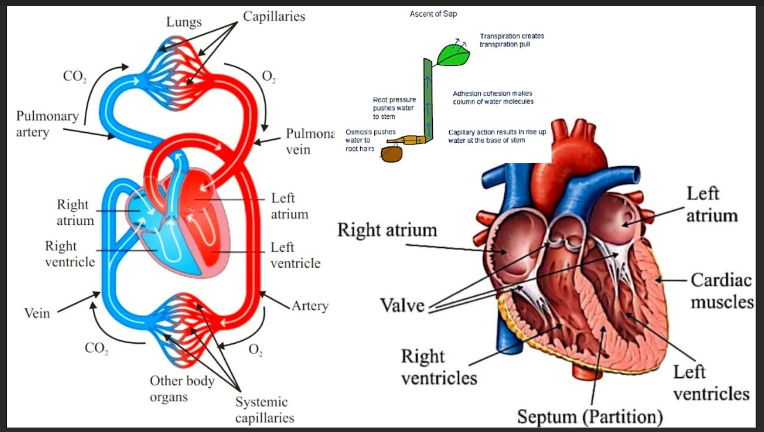
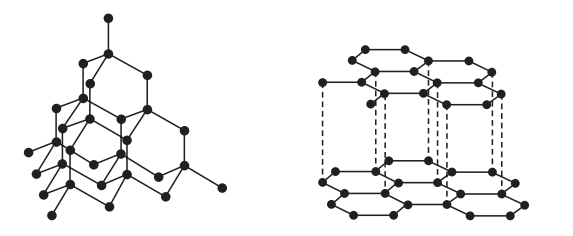
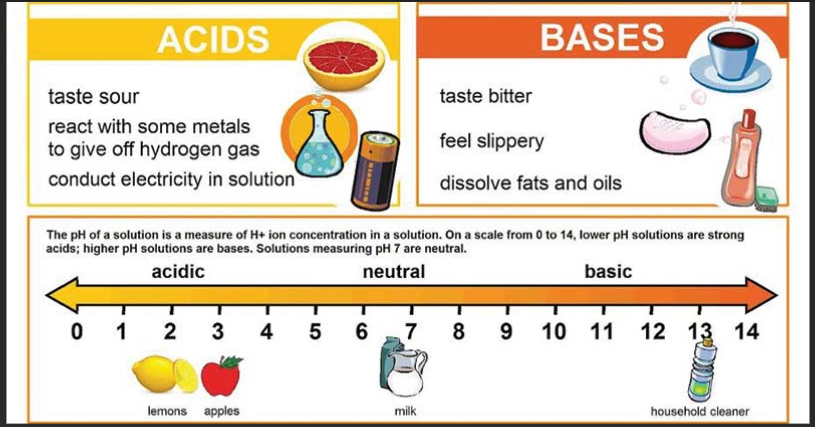
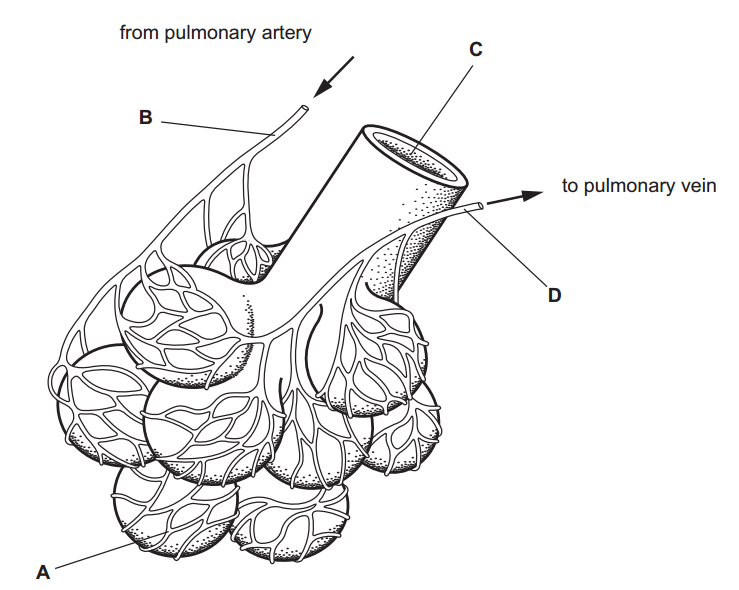
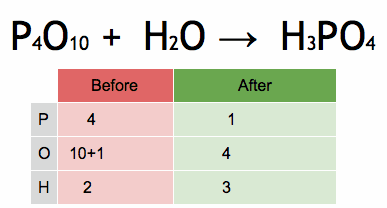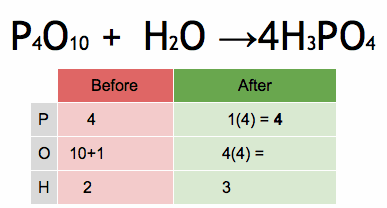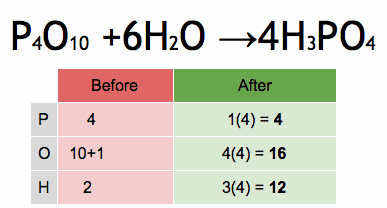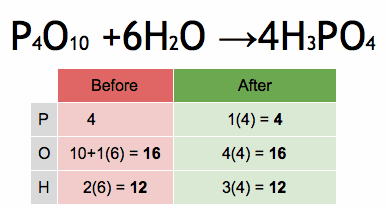
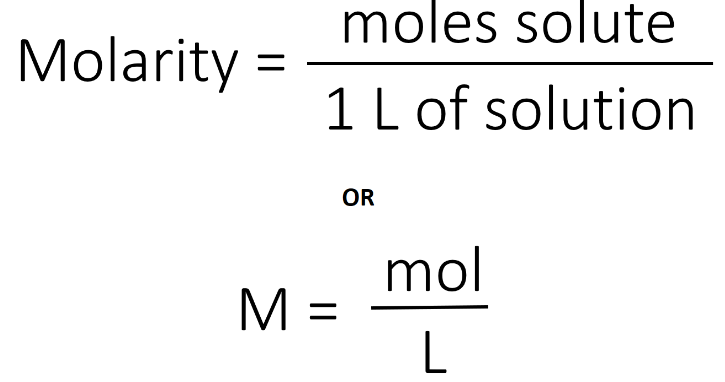1.Which of the following solutions would dissolve in water to form a solution that will turn red litmus blue?
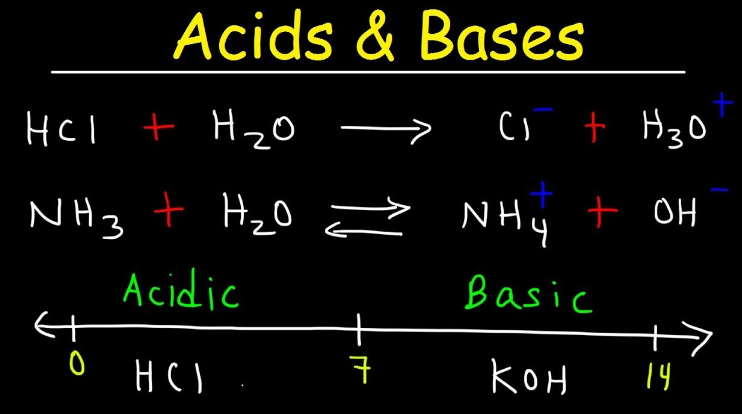
A. Sodium chloride
B. Sodium hydroxide
C. Sodium sulphate
D. sodium nitrate
2.Element M reacts with chlorine to form a compound with formular MCƖ4. The formular of oxide of M is A. M2O
B. MO
C. MO4
D. MO2
3.The following is the order of reactivity of metals with water from highest to lowest
A. Sodium magnesium lead copper
B. Magnesium sodium copper lead
C. Copper lead magnesium sodium
D. Lead copper sodium magnesium
4. The separation of ink substances by chromatography depends on the following
A. Size of chromatography paper
B. Solubilities of substance in a solvent
C. Freezing points of substances
D. Osmotic pressure of the solution of ink
5. The following carbonate decomposes to give a colourless gas which is alkaline
A. Calcium carbonate
B. Zinc carbonate
C. Potassium carbonate
D. Ammoniumcarbonate
6. Most metals react with dilute mineral acids to form
A. Hydrogen gas only
C. The salt of the metal only
B. Salt of metal and water
D. Salt of the metal and hydrogen gas
7. Which of the following substances below conducts electricity in solid state?
A. Graphite
B. Sulphur
C. Iodine
D. Phosphorus
8. Chlorine atom has electronic configuration 2:8:7. The electronic configuration of the ion of chlorine is
A. 2:8:7
B. 2:8:8
C. 2:8:6
D. 2:8:5
9. Brass is an alloy of;
A. Tin and copper
B. Zinc and copper
C. Lead and copper
D. Aluminum and copper
10. Which one of the following mixtures is best separated by chromatography
A. Ink
B. Crude petroleum
C. Water and oil
D. Water and ethanol
11. Atoms of elements in the same group of the periodic table have the same number of
A. Outer shell electrons
B. Protons in the nucleus
C. Electrons outside the nucleus
D. Neutrons in the nucleus
12. The process in which water vapour is changed into de is called
A. Distillation
B. Efflorescence
C. Condensation
D. Evaporation
13.Rust is hydrated ……………………………………………………….
A. Iron oxide
B. Iron(iii)hydroxide
C. Iron(ii)oxide
D. Iron(ii)hydroxide