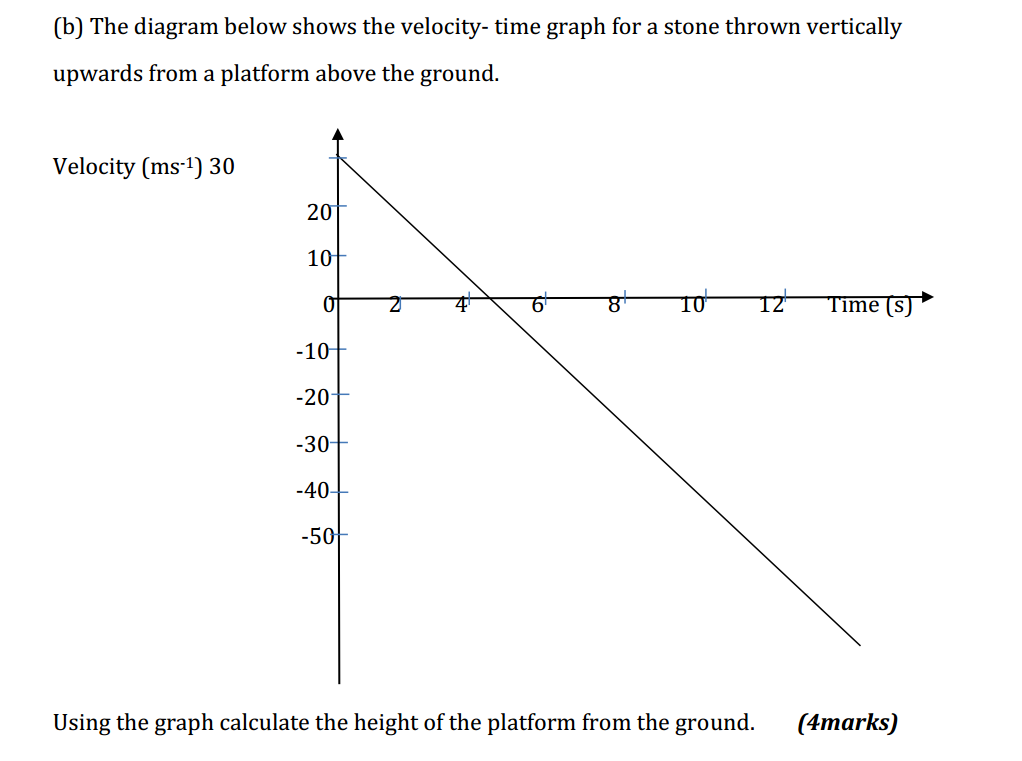
Scientific laws are statements that describe natural phenomena or relationships observed through repeated experimentation and observation. These laws are fundamental principles that summarize and explain the behavior of the natural world. They are derived from scientific theories and have been extensively tested and verified through empirical evidence. Scientific laws are typically expressed in concise mathematical or verbal form and are universally applicable within a specific domain or field of study. They provide a framework for understanding and predicting the behavior of physical, chemical, biological, and other natural processes. Scientific laws differ from scientific theories in that laws describe what happens in nature, whereas theories explain why or how phenomena occur based on underlying principles and mechanisms. Laws are considered more general and fundamental, whereas theories provide more detailed and comprehensive explanations. Scientific laws are subject to revision or refinement as new evidence or advancements in scientific understanding emerge. However, they have stood the test of time and are considered foundational concepts in their respective fields. Examples of well-known scientific laws include Newton's laws of motion, the law of conservation of energy, the laws of thermodynamics, and the law of gravity. These laws provide a framework for understanding and predicting the behavior of objects in motion, the transformation of energy, and the interaction of forces in the universe. Overall, scientific laws serve as fundamental principles that summarize and describe natural phenomena, providing a basis for scientific understanding and further exploration.
Here are some examples:
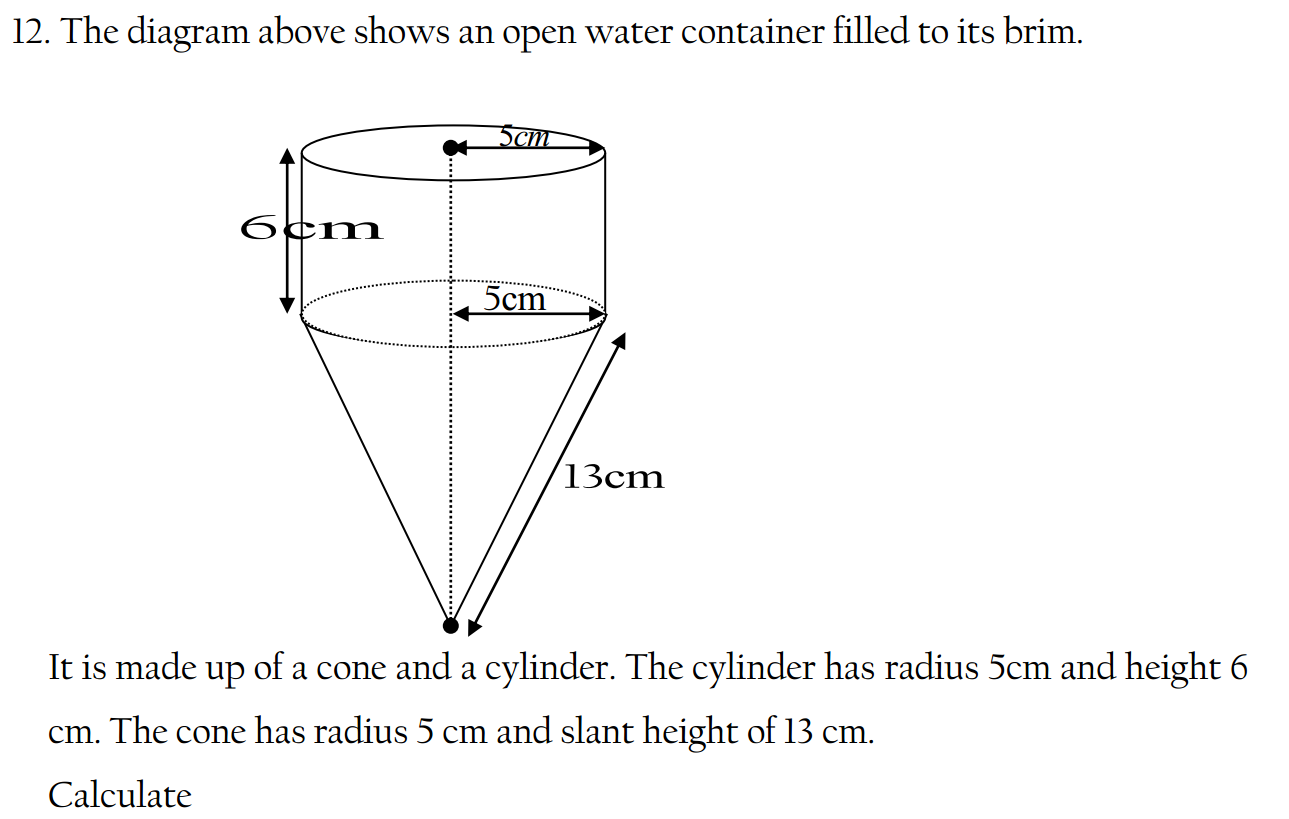
- Newton’s Laws of Motion: a. Newton’s First Law of Motion (Law of Inertia): An object at rest tends to stay at rest, and an object in motion tends to stay in motion with the same speed and in the same direction unless acted upon by an external force. b. Newton’s Second Law of Motion: The acceleration of an object is directly proportional to the net force acting on it and inversely proportional to its mass. This can be mathematically represented as F = ma, where F is the force, m is the mass, and a is the acceleration. c. Newton’s Third Law of Motion: For every action, there is an equal and opposite reaction.
- Law of Universal Gravitation: Every particle in the universe attracts every other particle with a force directly proportional to the product of their masses and inversely proportional to the square of the distance between them. This law is described by the equation F = G(m1m2/r^2), where F is the gravitational force, G is the gravitational constant, m1 and m2 are the masses of the two objects, and r is the distance between their centers.
- Laws of Thermodynamics: a. First Law of Thermodynamics (Law of Conservation of Energy): Energy cannot be created or destroyed in an isolated system. It can only be transformed from one form to another or transferred between systems. b. Second Law of Thermodynamics: The entropy (measure of disorder) of an isolated system tends to increase over time. It states that natural processes tend to move towards a state of greater disorder or randomness. c. Third Law of Thermodynamics: As the temperature approaches absolute zero, the entropy of a pure crystalline substance becomes zero.
- Law of Conservation of Mass-Energy: The total mass-energy in a closed system remains constant over time. This principle is derived from Einstein’s theory of relativity, which shows the equivalence of mass and energy.
- Ohm’s Law: The current flowing through a conductor between two points is directly proportional to the voltage across the two points and inversely proportional to the resistance of the conductor. It is expressed by the equation V = IR, where V is voltage, I is current, and R is resistance.
- Law of Conservation of Momentum: In a closed system, the total momentum before an event is equal to the total momentum after the event, provided no external forces are acting on the system.
These are just a few examples of common scientific laws. There are many more laws and principles that govern various branches of science, including chemistry, biology, and astronomy, among others. Each of these laws represents fundamental principles that help explain and predict the behavior of the natural world.
pepabank