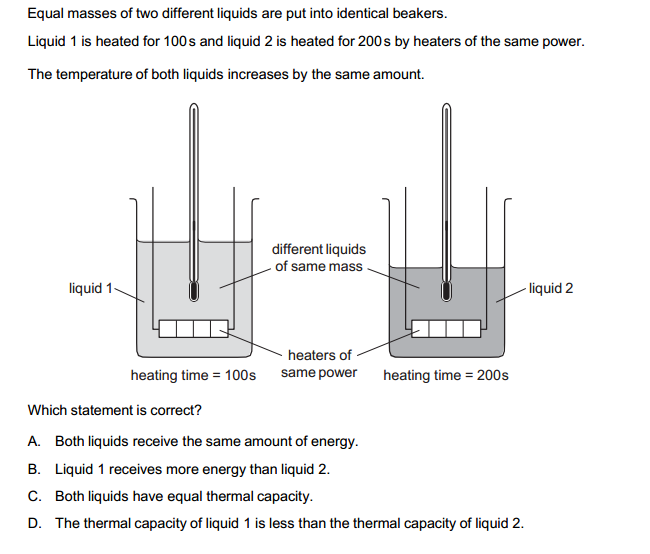
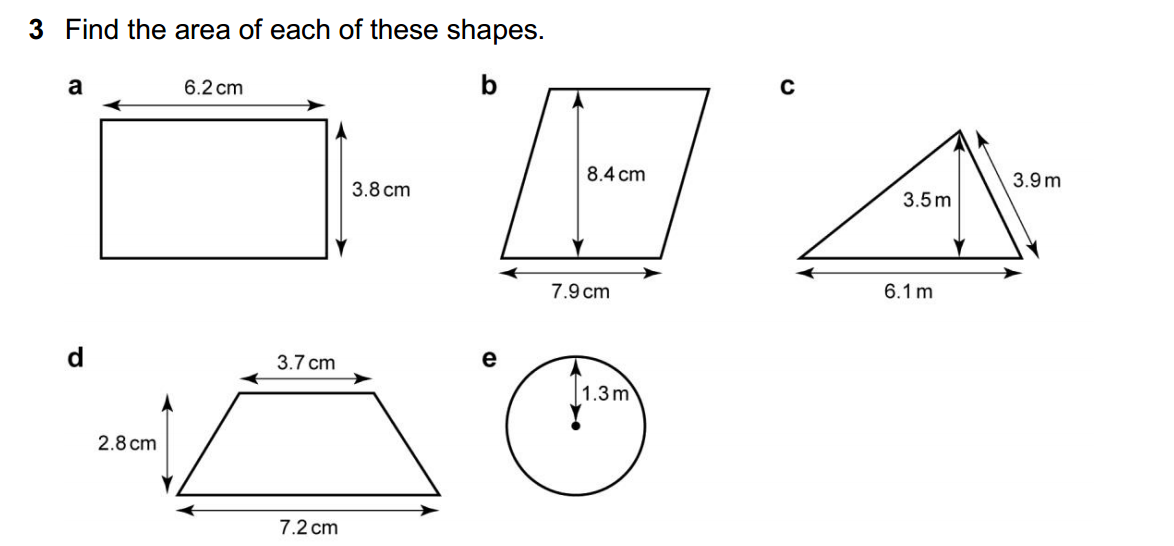
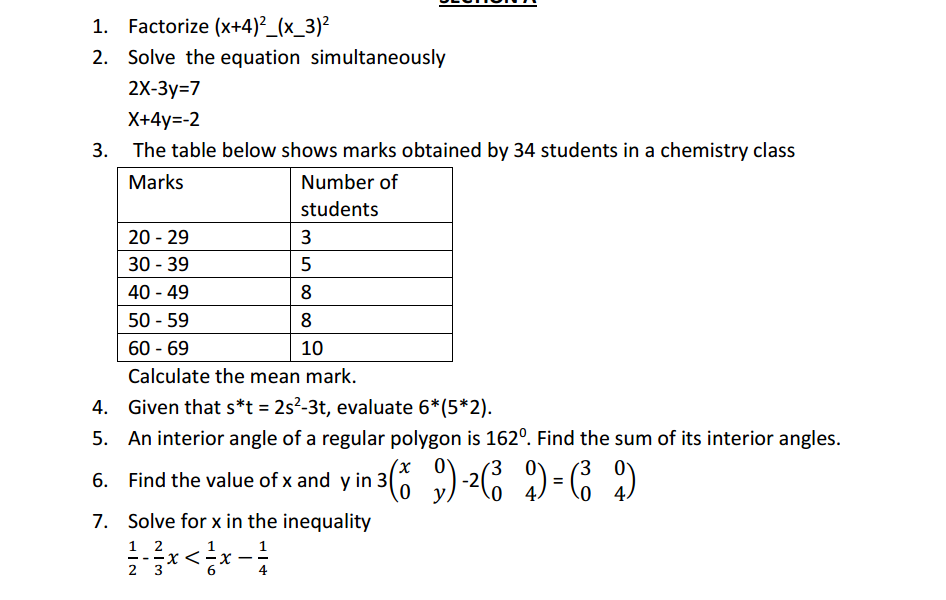
Acidity and basicity are fundamental concepts in chemistry that describe the properties of substances and their behavior in aqueous (water-based) solutions. These concepts are often associated with the pH scale, which measures the concentration of hydrogen ions (H⁺) in a solution. Here’s an overview of acidity and basicity:
- Acidity:
- Definition: Acidity is a measure of the concentration of hydrogen ions (H⁺) in a solution. The more H⁺ ions present, the more acidic the solution. Acids are substances that can donate H⁺ ions to the solution.
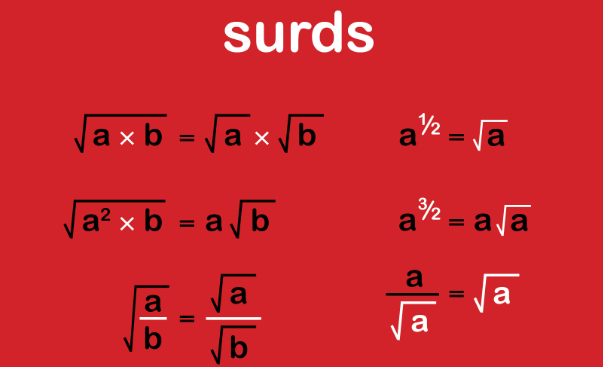
- pH Scale: The pH scale is used to quantify acidity. It ranges from 0 to 14, with 7 as the neutral point. Values less than 7 indicate acidity, with lower numbers indicating stronger acidity.
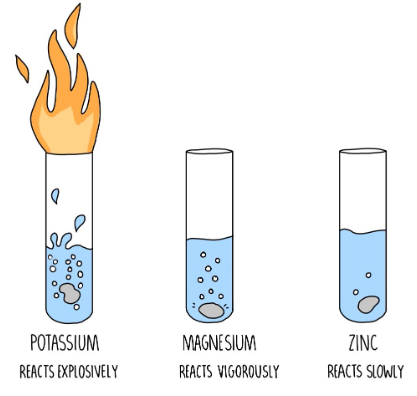
- Examples of Acids: Examples of common acids include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and acetic acid (CH₃COOH).
- Properties of Acids: Acids can react with bases to form salts and water. They can also corrode metals and have a sour taste (e.g., citric acid in citrus fruits).
- Basicity:
- Definition: Basicity is the measure of the concentration of hydroxide ions (OH⁻) in a solution. Bases are substances that can either directly release OH⁻ ions or accept H⁺ ions to produce OH⁻ ions in the solution.
- pH Scale: On the pH scale, values greater than 7 indicate basicity, with higher numbers indicating stronger basicity.
- Examples of Bases: Examples of common bases include sodium hydroxide (NaOH), ammonia (NH₃), and sodium bicarbonate (NaHCO₃).
- Properties of Bases: Bases can neutralize acids, forming salts and water. They can also feel slippery to the touch (e.g., soap) and have a bitter taste.
Acids and bases often interact in a way that’s described by the Brønsted-Lowry theory. In this theory, an acid is a substance that can donate a proton (H⁺ ion), while a base is a substance that can accept a proton. This definition is more general than the traditional H⁺ and OH⁻ definitions and encompasses a broader range of chemical reactions.
The relative strength of acids and bases can be compared using pKa values (for acids) and pKb values (for bases), which are related to the equilibrium constants for proton transfer reactions. Strong acids have low pKa values, while strong bases have low pKb values. Understanding the concepts of acidity and basicity is crucial in various fields of chemistry and biology, as they underlie chemical reactions, enzymatic processes, and the behavior of many chemical compound.
Discussion Questions on Acids and bases.
Acids:
- What is the definition of an acid according to the Brønsted-Lowry theory?
- Which gas dissolves in water to form a strong acid?
- What is the common name for hydrochloric acid?
- Which acid is commonly found in citrus fruits?
- Which of the following is a weak acid: HCl, H2SO4, or CH3COOH?
- What’s the pH of a solution with a high concentration of H+ ions?
- What’s the general taste of acidic substances?
- Which gas is produced when an acid reacts with a metal?
- What color does litmus paper turn in the presence of an acid?
- What are the properties of an acid when mixed with a base?
Bases:
- What is the definition of a base according to the Brønsted-Lowry theory?
- What is the common name for sodium hydroxide?
- Which base is commonly found in household cleaning products?
- What is the pH of a solution with a high concentration of OH- ions?
- What’s the taste of basic substances?
- Which of the following is a weak base: NaOH, NH3, or KOH?
- What color does litmus paper turn in the presence of a base?
- Which gas is produced when a base reacts with ammonium salts?
- What happens to the properties of a base when mixed with an acid?
- What’s the formula for the bicarbonate ion?
Strength and Concentration:
- How is the strength of an acid typically measured?
- What does a low pKa value indicate for an acid?
- Define the term “concentration” in the context of acids and bases.
- What is the pH of a neutral solution?
- How is the strength of a base typically measured?
- What is the pOH of a solution with a high concentration of OH- ions?
- Describe the relationship between pH and pOH.
- What does a low pKb value indicate for a base?
- Differentiate between strong and weak acids.
- Differentiate between strong and weak bases.
Reactions and Equations:
- When an acid reacts with a base, what compounds are typically formed?
- What’s the general term for a reaction between an acid and a base?
- What’s the product when HCl reacts with NaOH?
- What’s the product when H2SO4 reacts with KOH?
- Define neutralization in the context of acid-base reactions.
- What’s the name of the reaction when a carbonate reacts with an acid?
- What’s the product when acetic acid reacts with sodium bicarbonate?
- Define the term “salt” in the context of acid-base reactions.
- What’s the product when phosphoric acid reacts with calcium hydroxide?
- What is the role of an indicator in acid-base titrations?