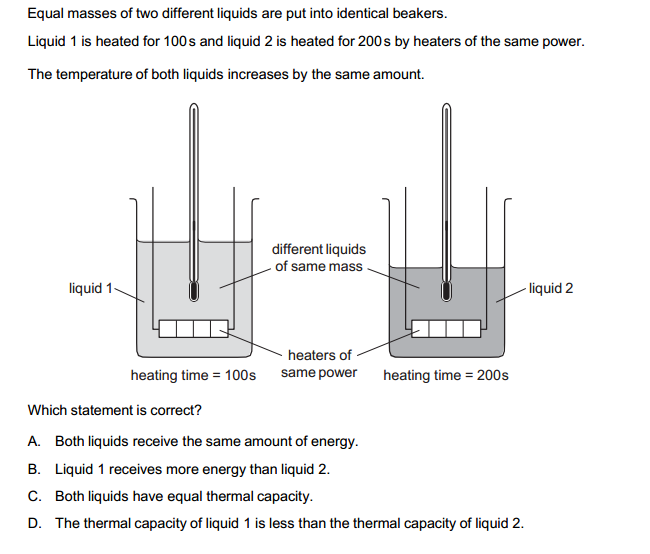
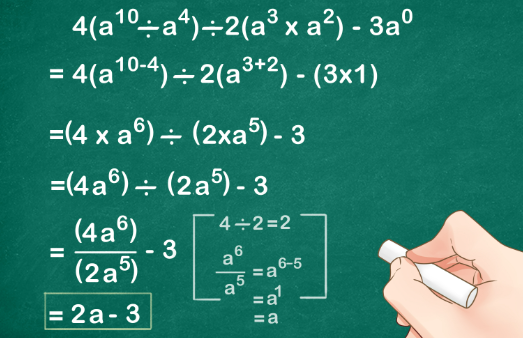No, heat and temperature are not the same, although they are related concepts.
Heat refers to the transfer of energy between objects or systems due to a difference in temperature. It is a form of energy transfer that occurs from an object at a higher temperature to an object at a lower temperature. Heat can be transferred through various mechanisms, such as conduction, convection, and radiation.
Temperature, on the other hand, is a measure of the average kinetic energy of the particles in a substance or system. It quantifies the “hotness” or “coldness” of an object or environment. Temperature is typically measured using a scale such as Celsius, Fahrenheit, or Kelvin.
In simpler terms, heat is the energy that is transferred, while temperature is a measure of the average energy of the particles within a system.
It’s important to note that heat and temperature are related but distinct concepts. Two objects can have the same temperature but different amounts of heat, and vice versa. For example, a cup of hot tea and a swimming pool may have the same temperature, but the cup of tea contains much less heat due to its smaller size.
I hope this clarifies the difference between heat and temperature. If you have any further questions, feel free to ask in comments.