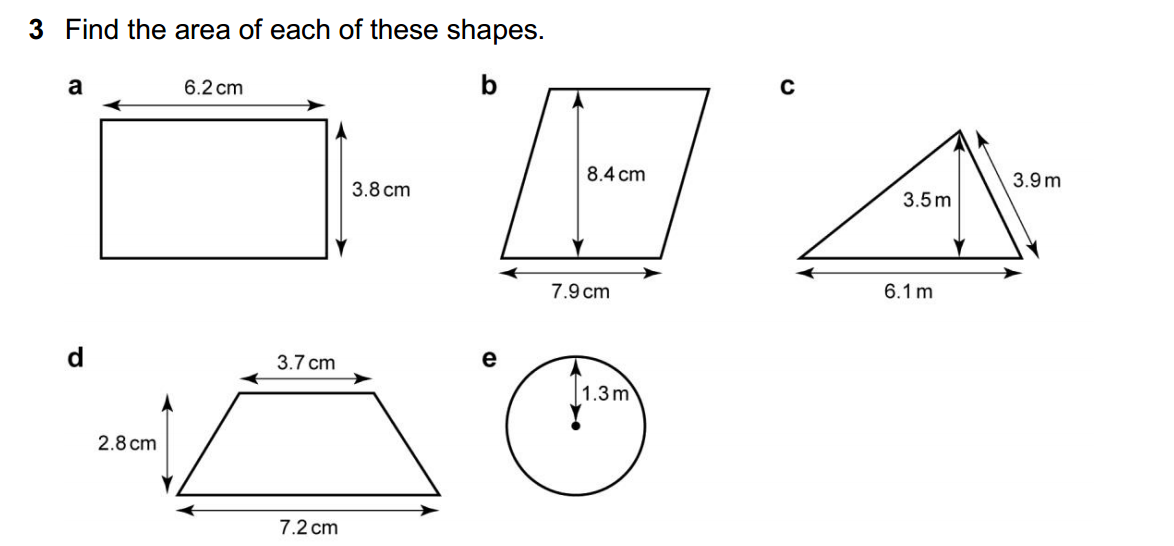
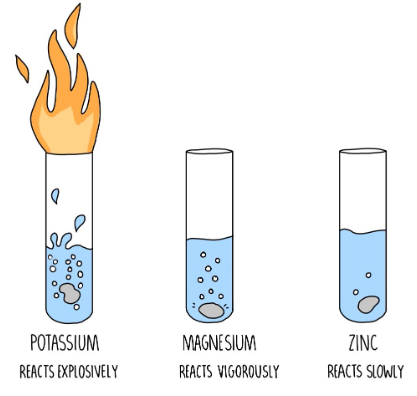
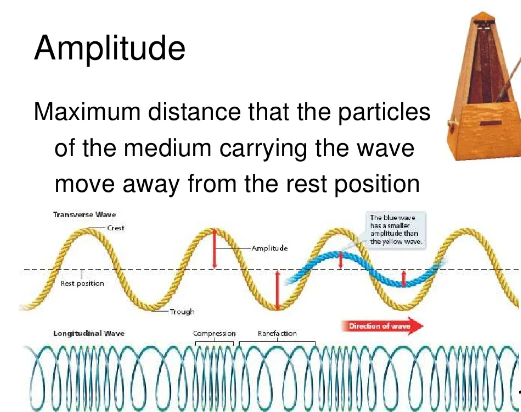
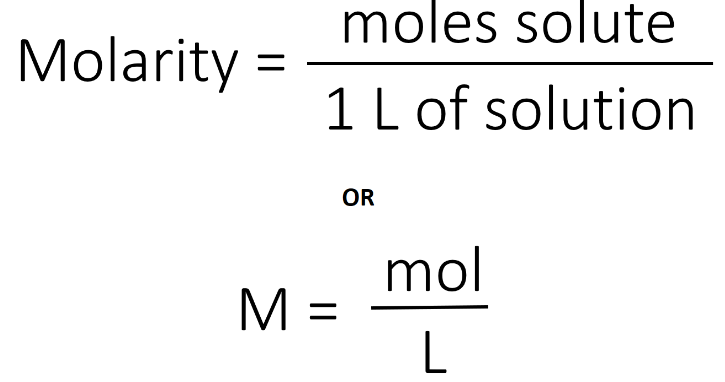Balancing chemical equations is an essential skill in chemistry, and it ensures that the law of conservation of mass is upheld in chemical reactions. While it may seem challenging at first, there is a systematic and straightforward approach to balancing chemical equations. Here’s a step-by-step method to easily balance chemical equations:
Step 1: Write the Unbalanced Equation
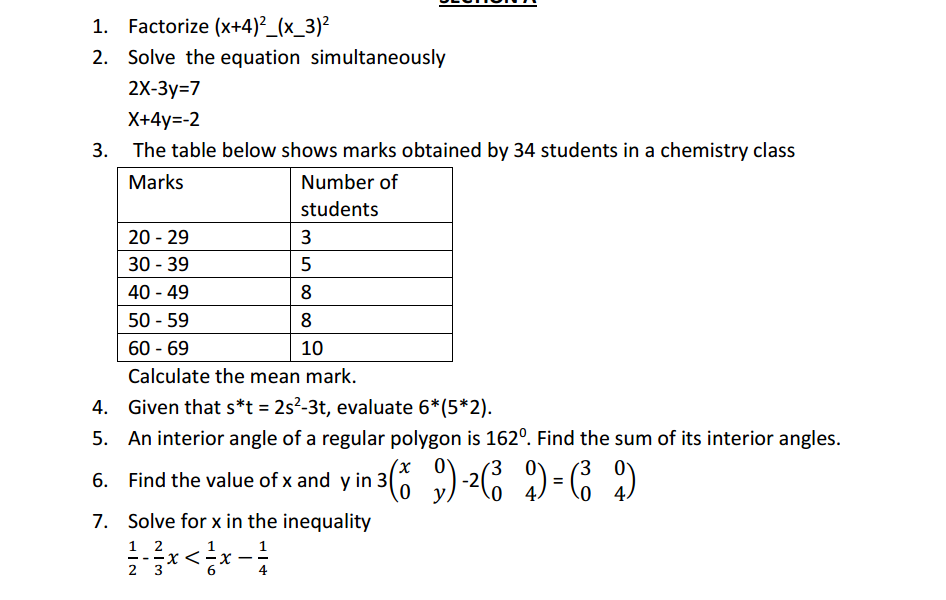
Start by writing the chemical equation for the reaction. Ensure you have the correct chemical formulas and that the equation represents the reaction accurately.
Step 2: Count the Atoms on Each Side
Count the number of atoms of each element on both the reactant and product sides of the equation. Make a list or table to keep track of the counts.
Step 3: Begin with the Most Complex Compound
Start by balancing the compound with the most elements in it. This is typically a compound that appears on both sides of the equation. Often, this is a compound that contains an element that occurs only once on each side.
Step 4: Use Coefficients
Add coefficients (whole numbers) in front of the compounds to balance the equation. Do not change the subscripts within the chemical formulas; only add coefficients to the compounds.
Step 5: Recount the Atoms
After adding coefficients to one compound, recount the number of atoms of each element on both sides of the equation. Make adjustments as necessary.
Step 6: Repeat and Work Methodically
Continue to balance the equation by focusing on one compound at a time. Balance compounds with more complex elements or those that appear in multiple places in the equation. Be systematic and patient.
Step 7: Keep the Ratios Simple
Strive to use the smallest possible whole number coefficients to balance the equation. Simple ratios make it easier to understand and interpret the reaction.
Step 8: Watch for Common Polyatomic Ions
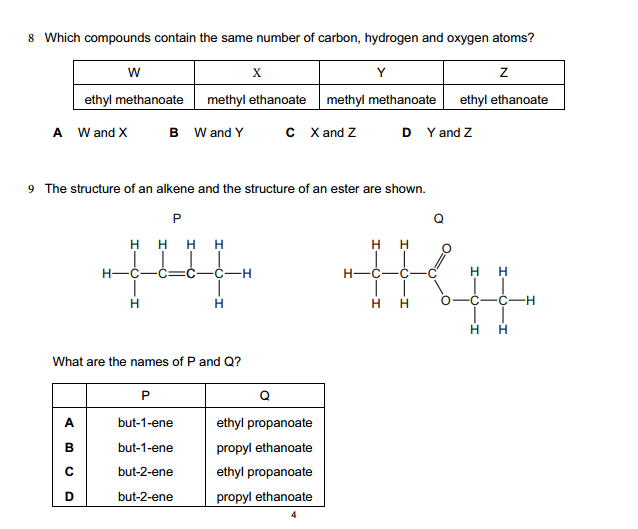
Some chemical reactions involve polyatomic ions (groups of atoms with a net charge). Treat them as single units and balance them accordingly.
Step 9: Check the Equation
After you think you’ve balanced the equation, double-check your work. Re-count the atoms on both sides to ensure they are equal.
Step 10: Balance Hydrogen and Oxygen Last
In many chemical reactions, it’s helpful to balance hydrogen and oxygen atoms last. This is because they often appear in multiple compounds and can throw off your balancing efforts if you address them too soon.
Step 11: Practice
Balancing chemical equations is a skill that improves with practice. The more equations you work on, the more proficient you’ll become.
Remember that while there is a systematic approach to balancing equations, it may take some trial and error to find the right coefficients. Don’t get discouraged if it doesn’t balance perfectly on your first attempt. With practice, you’ll become more efficient and accurate at balancing chemical equations.