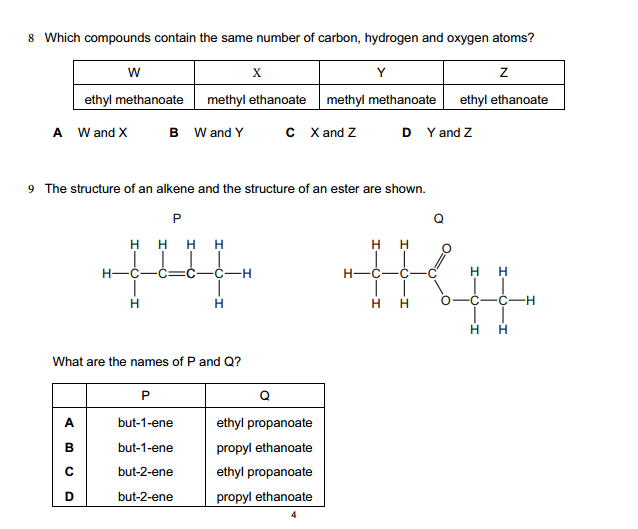
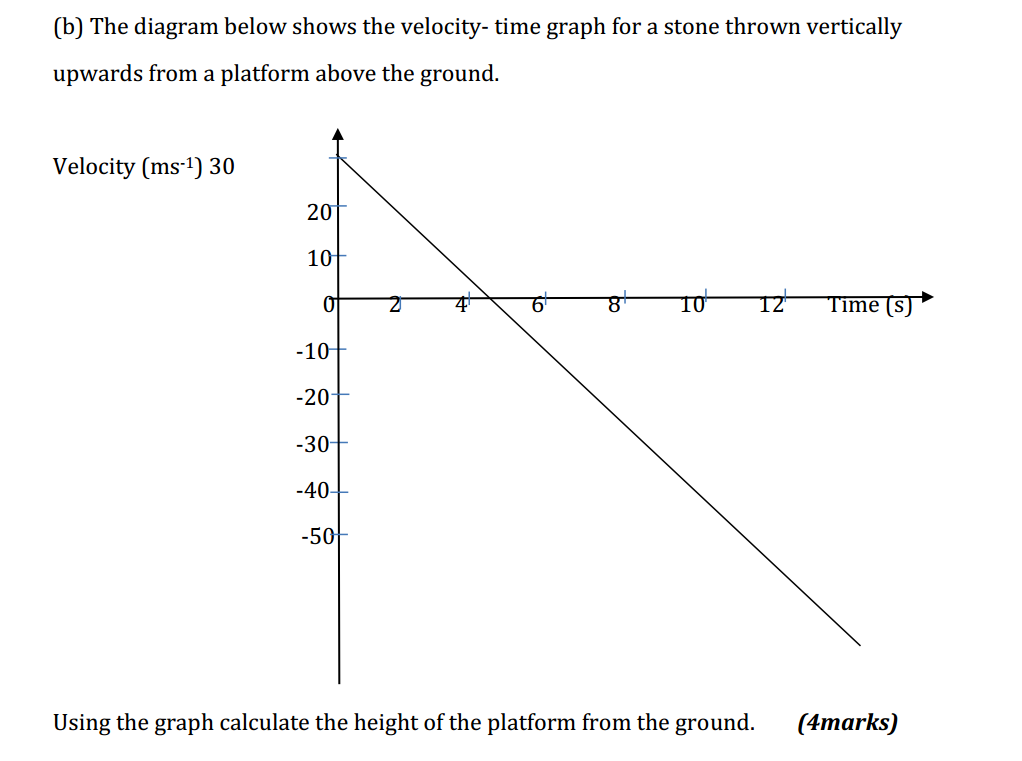
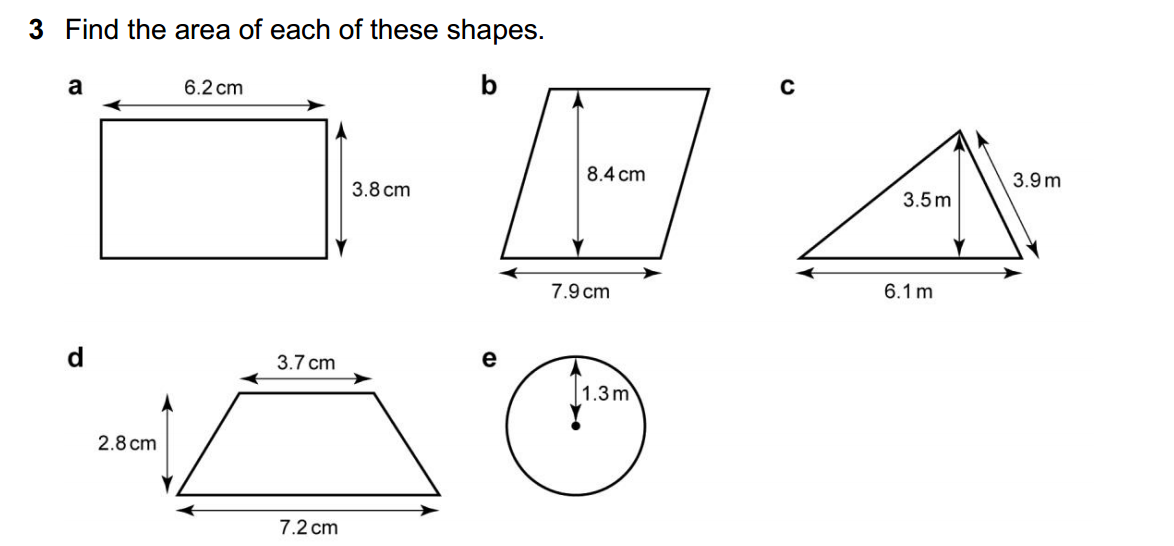
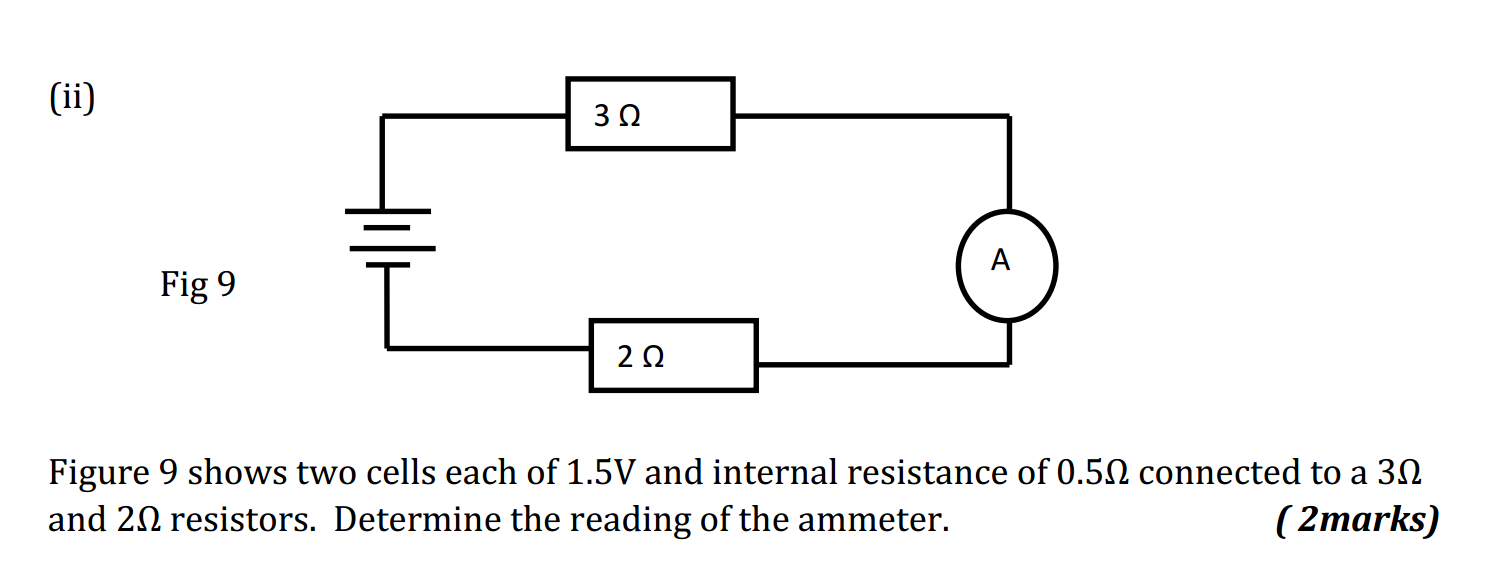
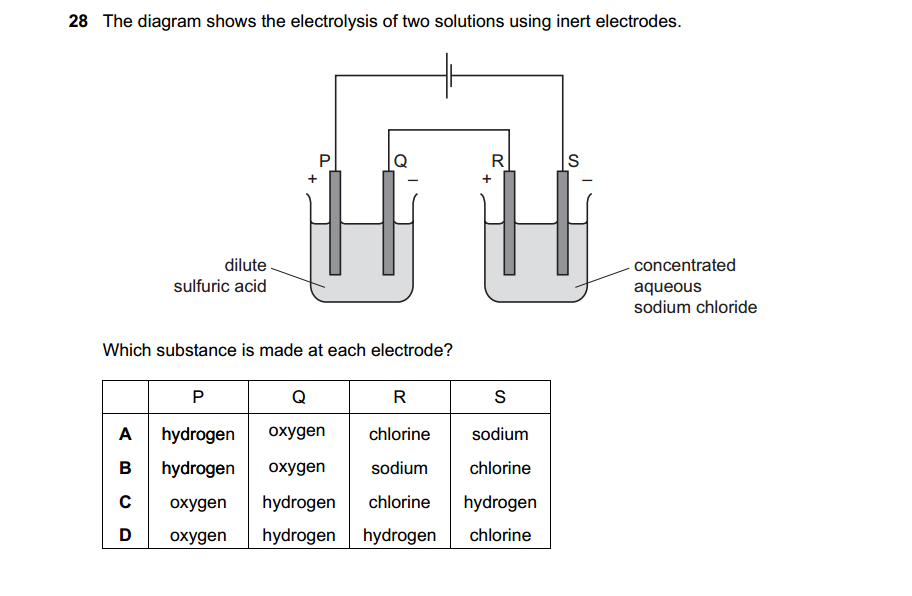
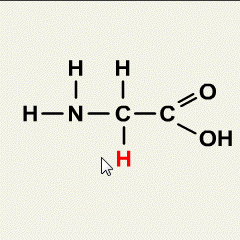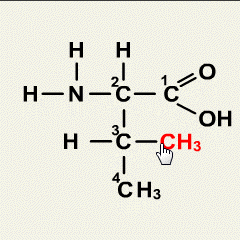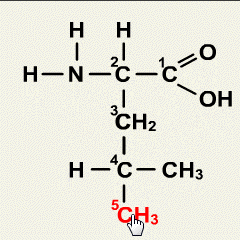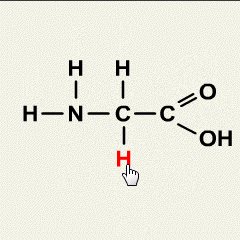
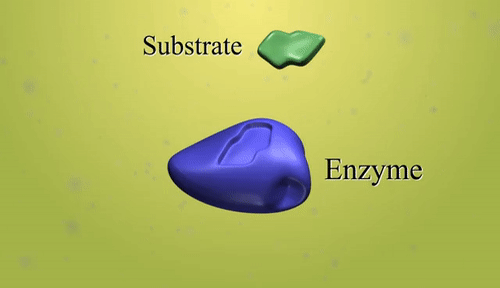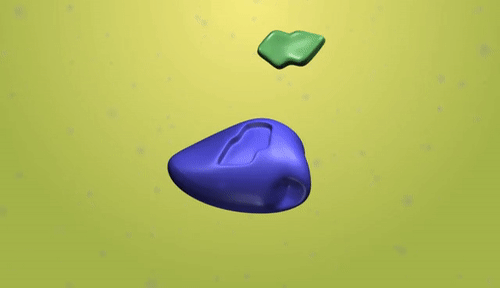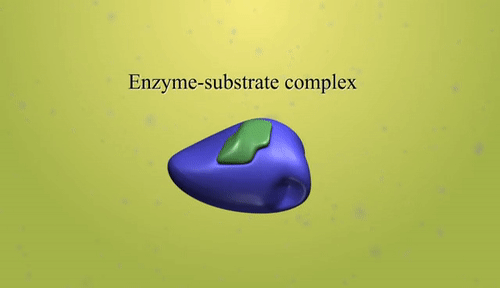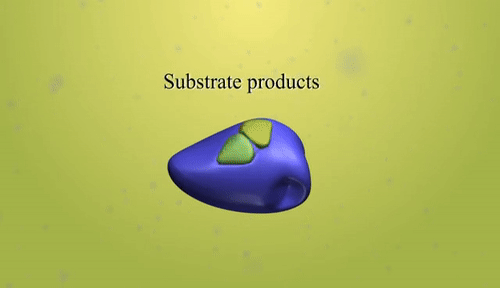
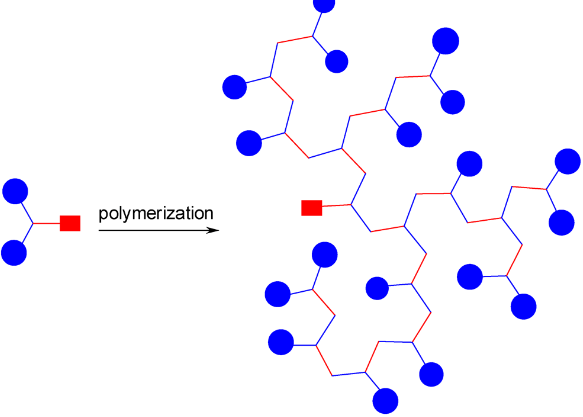
Synthetic polymerization refers to the process of chemically synthesizing polymers, which are large molecules composed of repeating subunits called monomers. This process involves the reaction of monomers to form covalent bonds and build a chain-like structure.
There are several methods of synthetic polymerization, but the most common ones are:
- Addition Polymerization: This process involves the repeated addition of monomers with unsaturated double or triple bonds, such as ethylene (monomer) to form polyethylene (polymer). This type of polymerization requires an initiator, which initiates the reaction and facilitates the growth of the polymer chain.
- Condensation Polymerization: In this process, monomers with functional groups, such as carboxylic acids and alcohols, react to form a polymer while eliminating a small molecule, such as water or alcohol. Examples of condensation polymers include nylon and polyester.
- Radical Polymerization: This type of polymerization involves the use of free radicals, which are highly reactive species with unpaired electrons. Free radicals initiate the polymerization reaction and propagate the growth of the polymer chain. Common examples of polymers produced through radical polymerization include polystyrene and polyvinyl chloride (PVC).
- Ring-Opening Polymerization: This process occurs when a cyclic monomer, such as lactide or caprolactam, opens up to form a linear polymer. Ring-opening polymerization is commonly used to produce polymers like polylactic acid (PLA) and nylon.
Synthetic polymerization plays a crucial role in the production of various everyday materials, including plastics, fibers, coatings, adhesives, and many other products. The specific polymerization method used depends on the desired properties of the polymer and the intended application
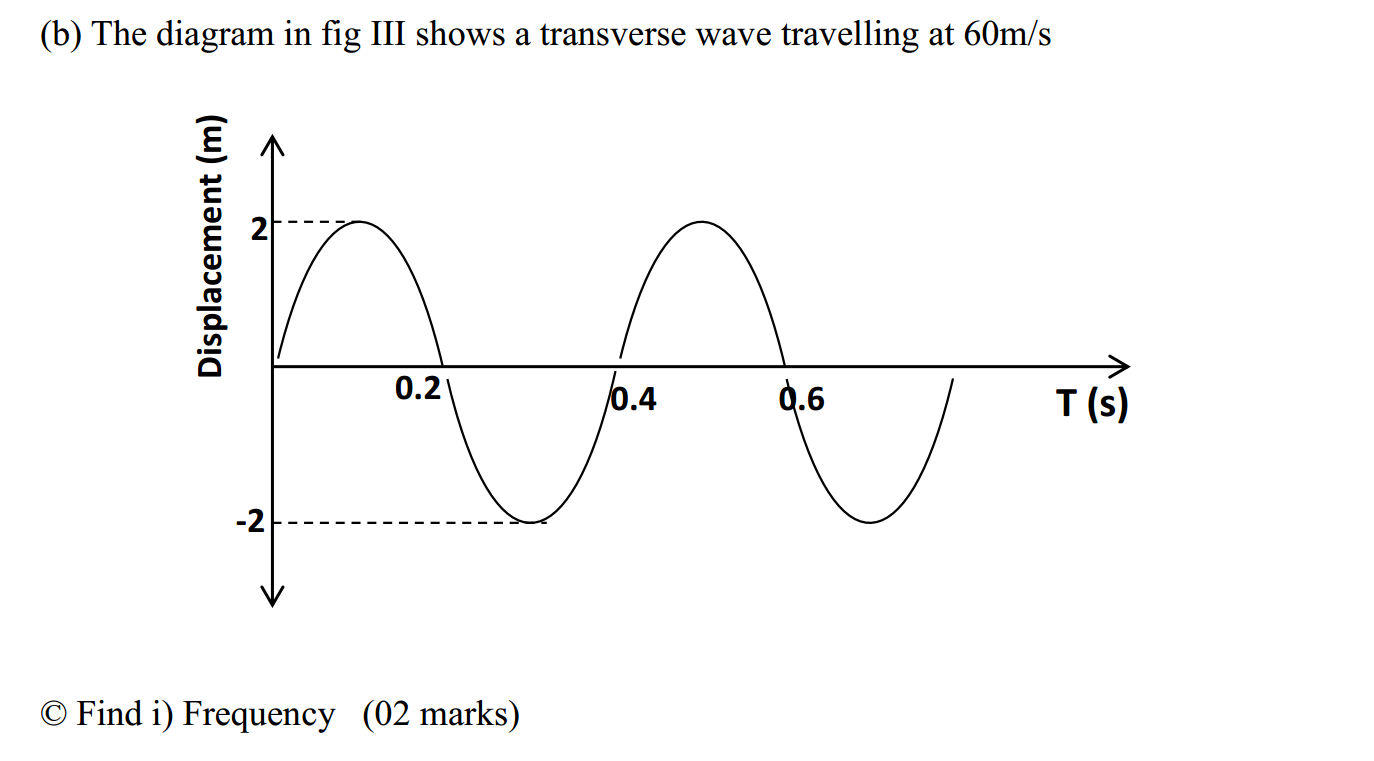
Click on any of the Image below to start the question slide on polymers…