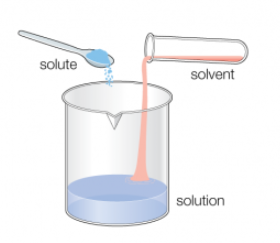
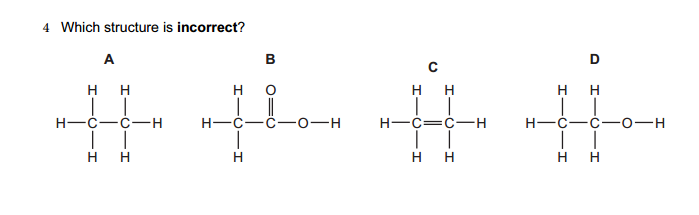
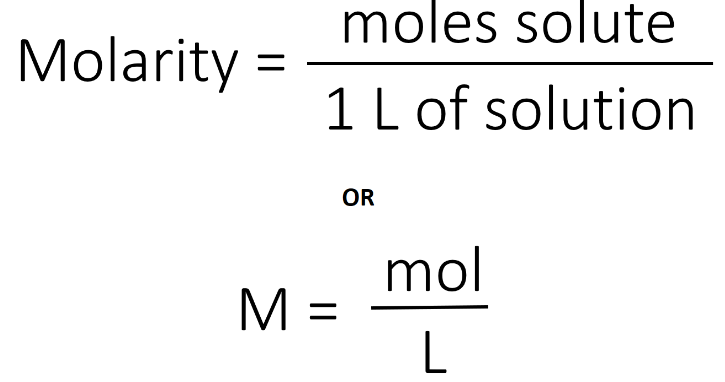Molarity, often denoted as “M,” is a fundamental concept in chemistry that describes the concentration of a solute (a substance being dissolved) in a solution (the resulting mixture). It is defined as the number of moles of solute present in one liter of solution. In other words, molarity measures the amount of a substance (in moles) per unit volume (in liters) of the solution.
Here’s a more detailed explanation of molarity:
Mathematical Representation: Molarity (M) is calculated using the following formula:

Units: Molarity is expressed in moles per liter (mol/L or M). For example, a solution with a molarity of 1 M contains one mole of solute in each liter of solution.
Key Points:
- Moles of Solute: To calculate molarity, you need to know the number of moles of the solute. This can be determined by measuring the mass of the solute and using its molar mass. For example, if you have 1 mole of sodium chloride (NaCl) in 1 liter of water, the solution has a molarity of 1 M.
- Volume of Solution: Molarity depends on the volume of the solution, so it’s important to specify the volume when expressing molarity. Be sure to use consistent units (usually liters) for volume to maintain accuracy.
- Concentration: Molarity is a measure of concentration. A higher molarity indicates a more concentrated solution, as it contains more solute in the same volume of solution.
- Dilution: Solutions with a higher molarity can be diluted to create solutions with lower molarity. This is often done in laboratory settings to create solutions of the desired concentration.
- Reaction Stoichiometry: Molarity is used in stoichiometry to determine the amounts of reactants and products in chemical reactions. It allows chemists to calculate the quantity of a substance needed or produced in a reaction.
- Standard Solution: In analytical chemistry, solutions with known molarity, known as standard solutions, are used for titration and calibration purposes.
Molarity is a fundamental concept in various branches of chemistry, including analytical chemistry, physical chemistry, and chemical engineering. It is essential for making solutions with known concentrations, performing chemical analyses, and understanding the behavior of solutes in solutions.