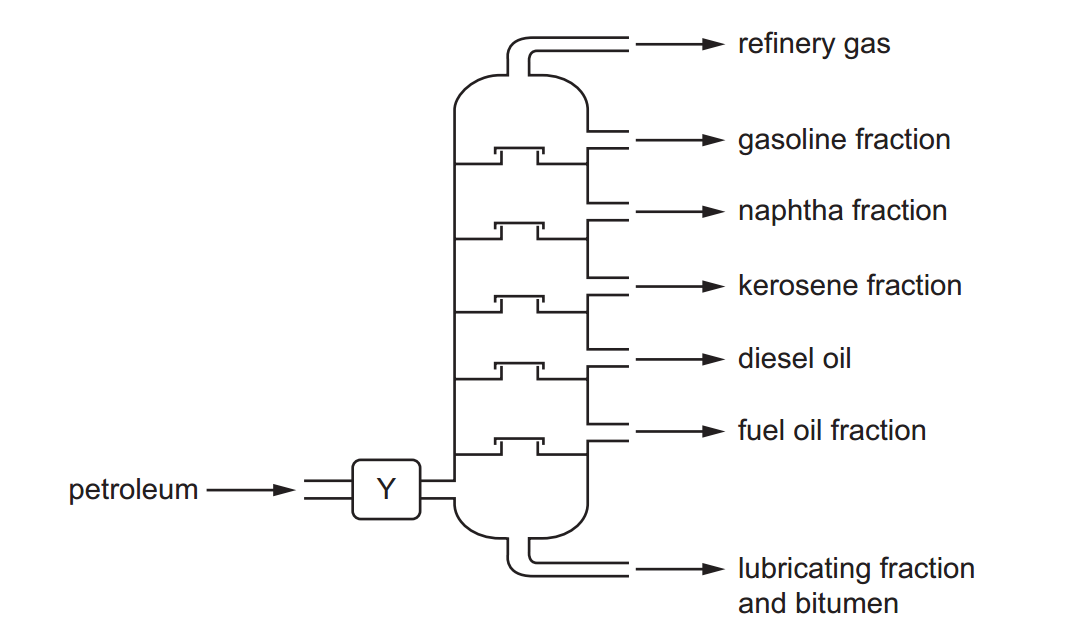
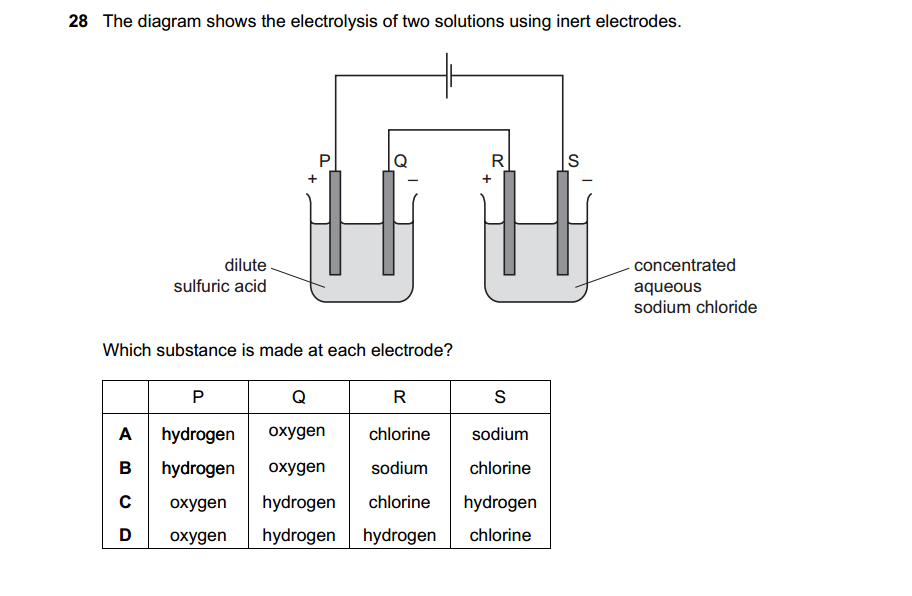
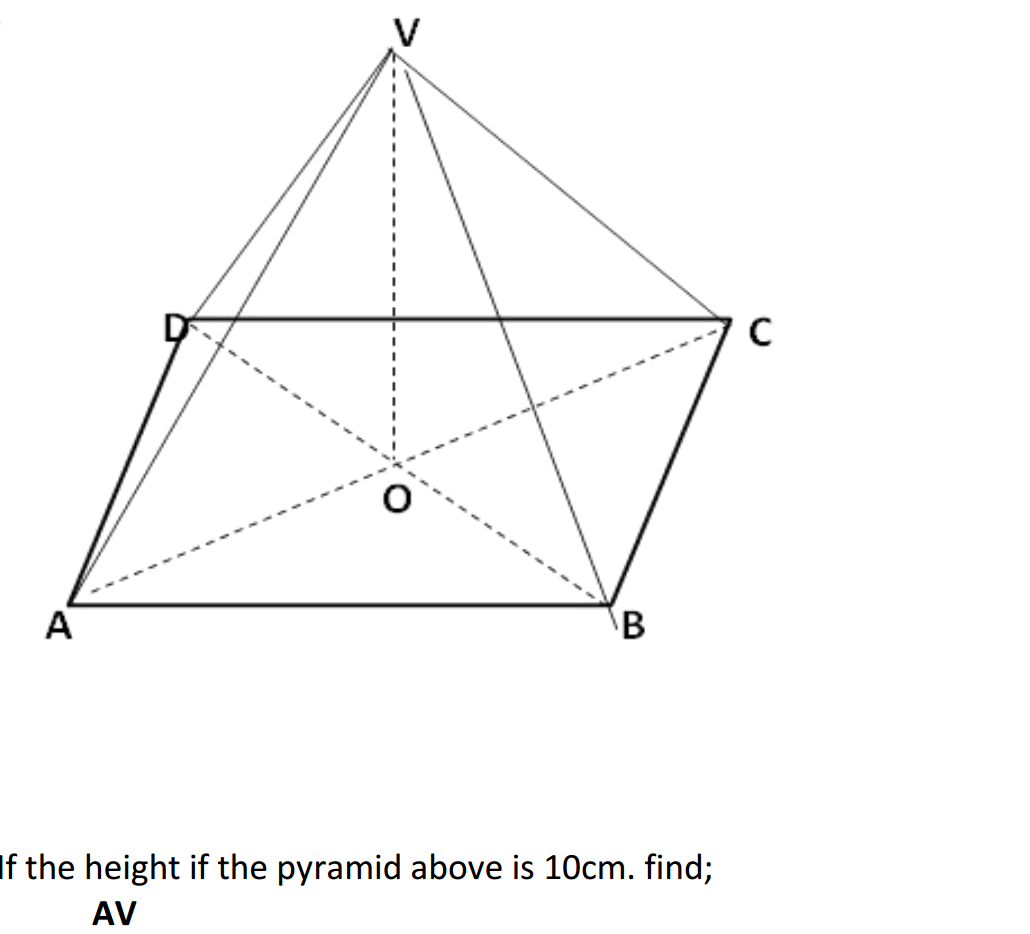
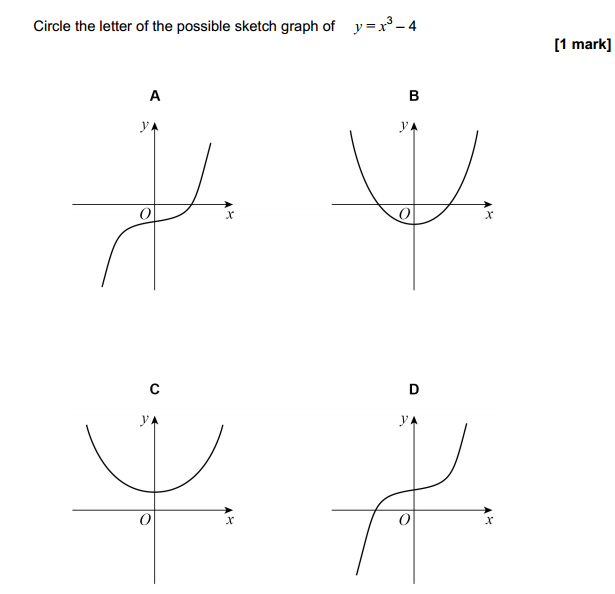
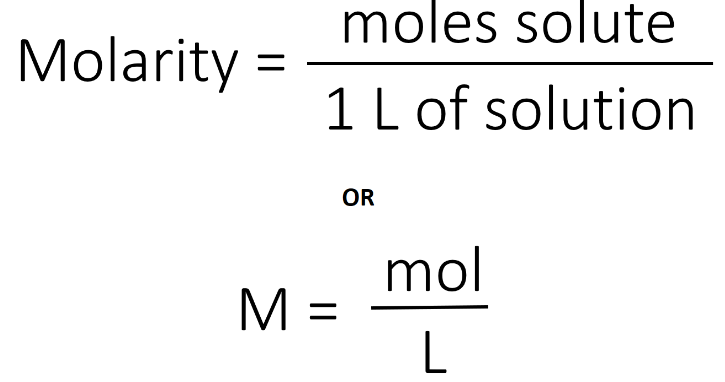Acids can be prepared through various methods depending on the specific acid required and the starting materials available. Here are some common methods for preparing acids:
- Direct Synthesis:
- Some acids can be prepared directly by combining the appropriate elements or compounds. For example:
- Hydrochloric acid (HCl) can be prepared by bubbling hydrogen gas through a solution of concentrated sulfuric acid.
- Nitric acid (HNO₃) can be prepared by reacting nitrogen dioxide (NO₂) with water.
- Some acids can be prepared directly by combining the appropriate elements or compounds. For example:
- Dissociation of Salts:
- Many acids can be prepared by the dissociation of their corresponding salts in water. For example:
- Hydrochloric acid (HCl) can be obtained by dissolving hydrogen chloride gas in water.
- Sulfuric acid (H₂SO₄) can be obtained by dissolving sulfur trioxide (SO₃) in water, followed by hydration to form sulfuric acid.
- Many acids can be prepared by the dissociation of their corresponding salts in water. For example:
- Oxidation of Non-Metals:
- Some acids can be prepared by oxidizing non-metallic elements or compounds. For example:
- Nitric acid (HNO₃) can be prepared by oxidizing ammonia (NH₃) with oxygen or air in the presence of a catalyst.
- Sulfuric acid (H₂SO₄) can be prepared by oxidizing sulfur dioxide (SO₂) to sulfur trioxide (SO₃), which is then dissolved in water to form sulfuric acid.
- Some acids can be prepared by oxidizing non-metallic elements or compounds. For example:
- Hydration of Gases:
- Certain acidic gases can be dissolved in water to form acids. For example:
- Carbon dioxide (CO₂) dissolves in water to form carbonic acid (H₂CO₃).
- Sulfur dioxide (SO₂) dissolves in water to form sulfurous acid (H₂SO₃).
- Certain acidic gases can be dissolved in water to form acids. For example:
- Biological Processes:
- Some acids can be produced through biological processes. For example:
- Citric acid, a common acid found in citrus fruits, is produced by certain microorganisms through fermentation.
- Some acids can be produced through biological processes. For example:
- Extraction from Natural Sources:
- Some acids can be extracted from natural sources. For example:
- Acetic acid, the main component of vinegar, can be obtained through fermentation of sugars by bacteria.
- Some acids can be extracted from natural sources. For example:
These are just a few methods for preparing acids. The choice of method depends on factors such as the desired acid, availability of starting materials, and scale of production. It’s important to follow proper safety precautions and procedures when working with acids, as they can be corrosive and hazardous.