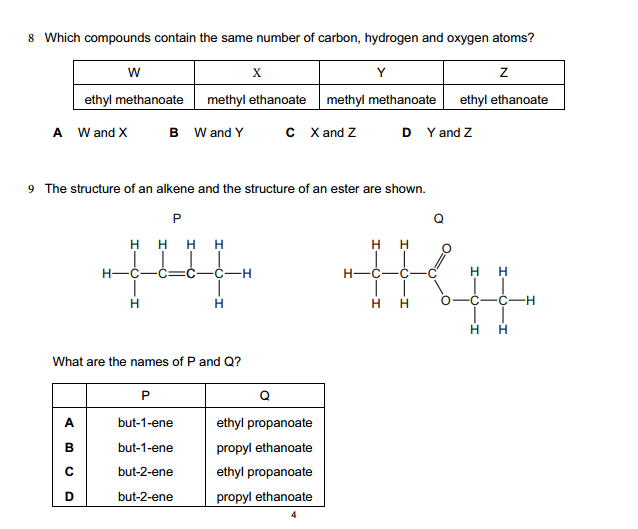
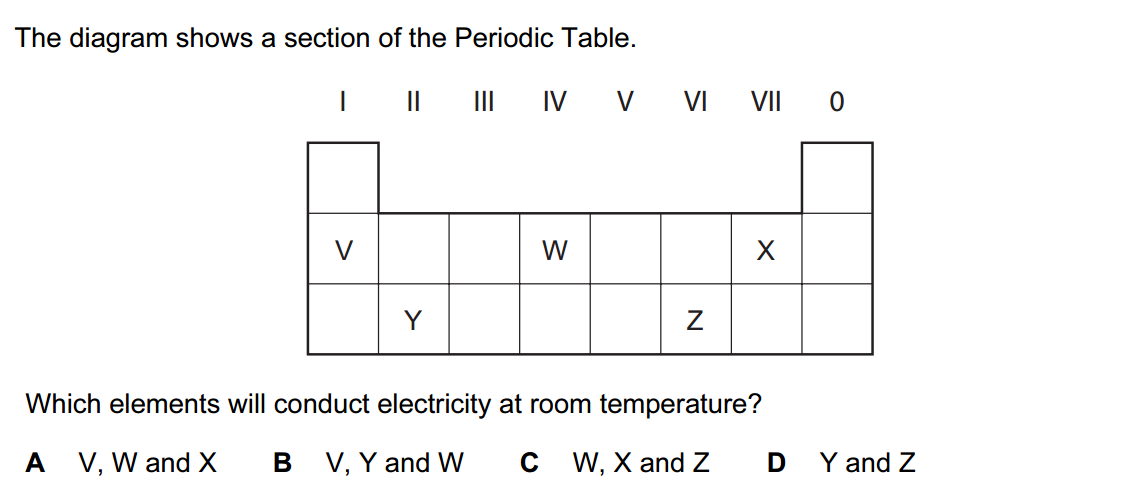
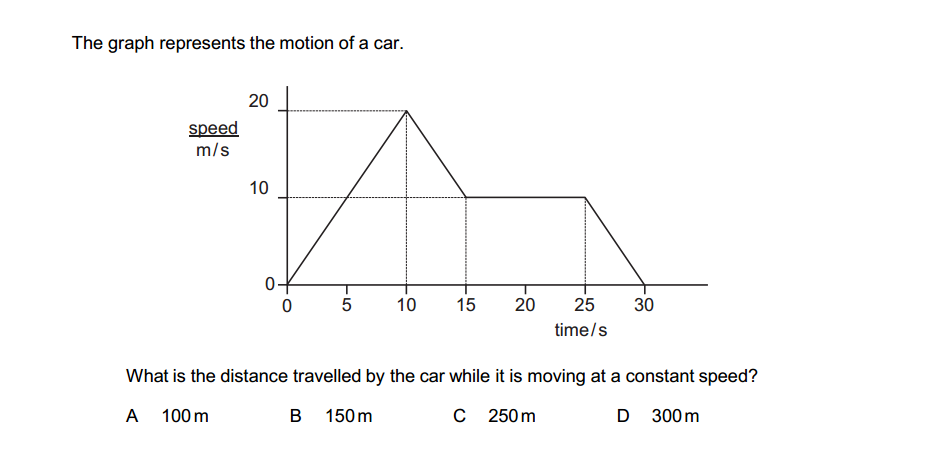
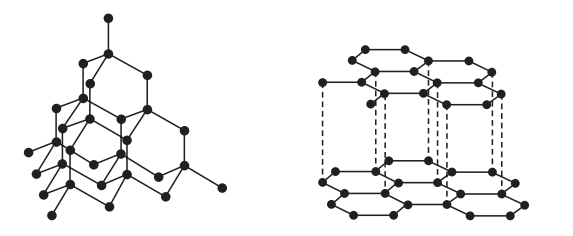
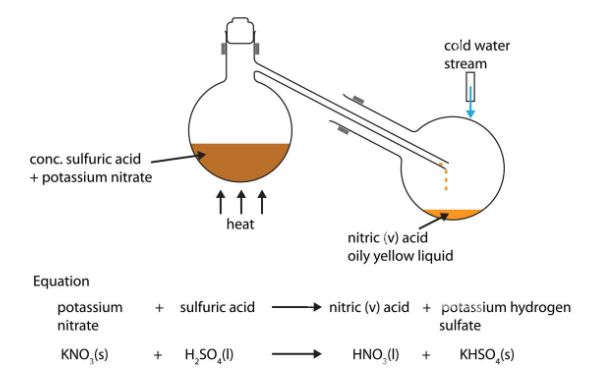
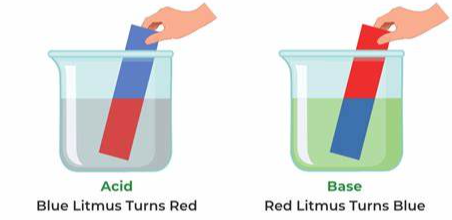
Inorganic chemistry is a branch of chemistry that focuses on the study of inorganic compounds, which are compounds that do not contain carbon-hydrogen (C-H) bonds. Instead, inorganic chemistry primarily deals with compounds of metals, minerals, and organometallic compounds, as well as the behavior of nonmetals. Here’s a brief introduction to inorganic chemistry:
Key Aspects of Inorganic Chemistry:
- Inorganic Compounds: Inorganic chemistry covers a vast array of compounds, including minerals, metals, metalloids, nonmetals, and their various combinations. These compounds often play critical roles in industries, materials science, and environmental processes.
- Coordination Chemistry: One of the central areas of inorganic chemistry is coordination chemistry, which focuses on the study of coordination compounds. These are complex compounds formed by the coordination of metal ions with ligands, such as water, ammonia, or organic molecules.
- Transition Metals: Transition metal chemistry is a prominent part of inorganic chemistry, as transition metals have unique properties and play essential roles in catalysis, electronic devices, and the biological functions of organisms.
- Organometallic Chemistry: Organometallic compounds contain metal-to-carbon bonds and are integral to industrial processes, including catalysis, as well as the synthesis of organic compounds.
- Main Group Chemistry: Inorganic chemistry also encompasses the chemistry of main group elements (non-transition metals), such as the alkali metals, alkaline earth metals, and various nonmetals like nitrogen, oxygen, and halogens.
- Descriptive Chemistry: A significant aspect of inorganic chemistry is descriptive chemistry, which involves classifying, identifying, and categorizing inorganic compounds based on their properties and behavior.
Applications of Inorganic Chemistry:
Inorganic chemistry has a wide range of practical applications, including:
- Catalysis: Understanding the behavior of inorganic compounds, especially transition metals, is crucial in catalysis, a process used in the production of chemicals, fuels, and pharmaceuticals.
- Materials Science: Inorganic compounds are essential in the development of materials, including superconductors, ceramics, and semiconductors, used in various technological applications.
- Environmental Chemistry: Inorganic chemistry plays a role in studying environmental processes, such as water purification, pollution control, and the geochemical behavior of elements in natural systems.
- Medicinal Chemistry: Inorganic compounds are used in medicinal chemistry to design drugs and study their interactions with biological systems.
- Geology and Earth Sciences: Inorganic chemistry is essential for understanding the composition and behavior of minerals, rocks, and the Earth’s crust.
- Nanotechnology: Inorganic nanomaterials have unique properties and are used in the development of nanotechnology-based applications.
Inorganic chemistry is a diverse field that bridges the gap between the fundamental understanding of the behavior of inorganic compounds and their wide-ranging practical applications in science and industry. It provides valuable insights into the properties and reactivity of non-organic substances, contributing to advancements in various fields of science and technology.